#1 FMEA RISK MANAGEMENT SYSTEM SOLUTION
Accelerate Your FMEA Risk Management with Visure.
Reduce the stress of inspections and audits by integrating Risk Management in a single system.
- Most cost-effective
- Access All Features
- 30-Day Trial


1,000+ Highly Regulated Organizations Trust Visure














What are Risk Management and FMEA? Why are they important?
Risk management is about foreseeing the unexpected and taking proactive measures to mitigate its potential adverse effects. The significance of risk management is manifold and extends across various sectors including business, finance, healthcare, and even personal decision-making.
Failure Mode and Effects Analysis (FMEA) is a structured methodology employed to analyze the potential failure modes of a system, product, or process and their potential consequences on performance, safety, and overall functionality. This approach minimizes the likelihood of costly failures, recalls, or safety incidents, ultimately contributing to improved efficiency, cost savings, and brand reputation.
- Safeguards Assets & Resources
- Eliminate, Calculate and Reduce Failures
- Enhances Product Quality & Resource Allocation
- Facilitates Innovation & Decision-Making
Integrate Risk-Based Thinking and FMEA into Your Requirements Management Process
Visure simplifies the process of building complex and high-quality products with verified and validated requirements to help you comply with applicable regulatory requirements by combining risk analysis and requirements management in a single solution.
Moreover, with Visure’s enterprise risk management software, you can easily capture, analyze, evaluate, and mitigate risks at each stage of the development process in one central location helping you avoid last-minute fixes and reworks.
- Easily Capture and Analyze Risks
- Automate Your Risk Mitigation Process
- Create Safety Requirements
- Effective Regulatory Compliance
- Gain Full Traceability & Collaboration
- Improve Product Quality
Streamline Your Product Development Process with All-in-One Risk Management & FMEA System

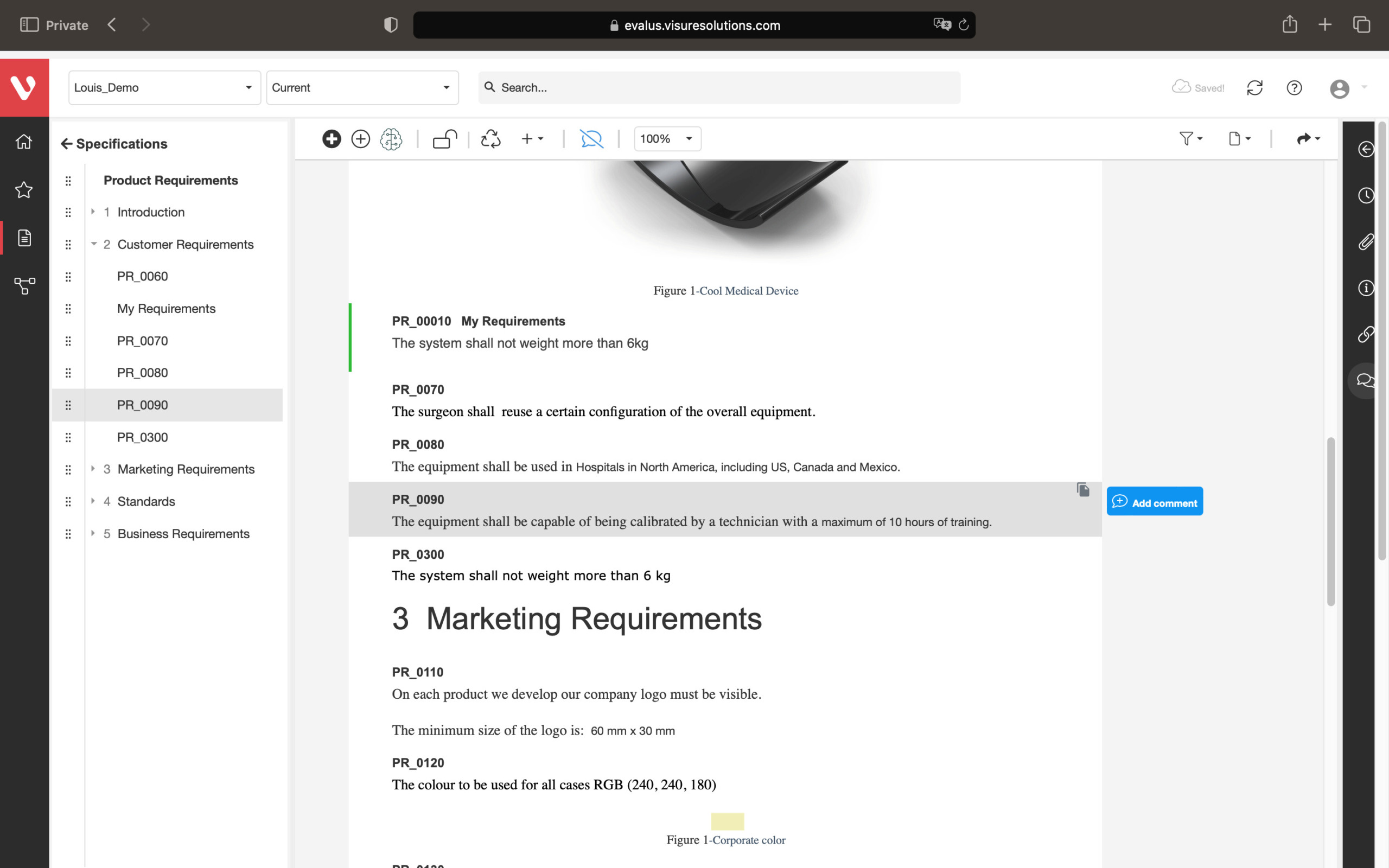
Achieve a balance between atomic requirements management and traceability, all while maintaining a familiar document-style approach.

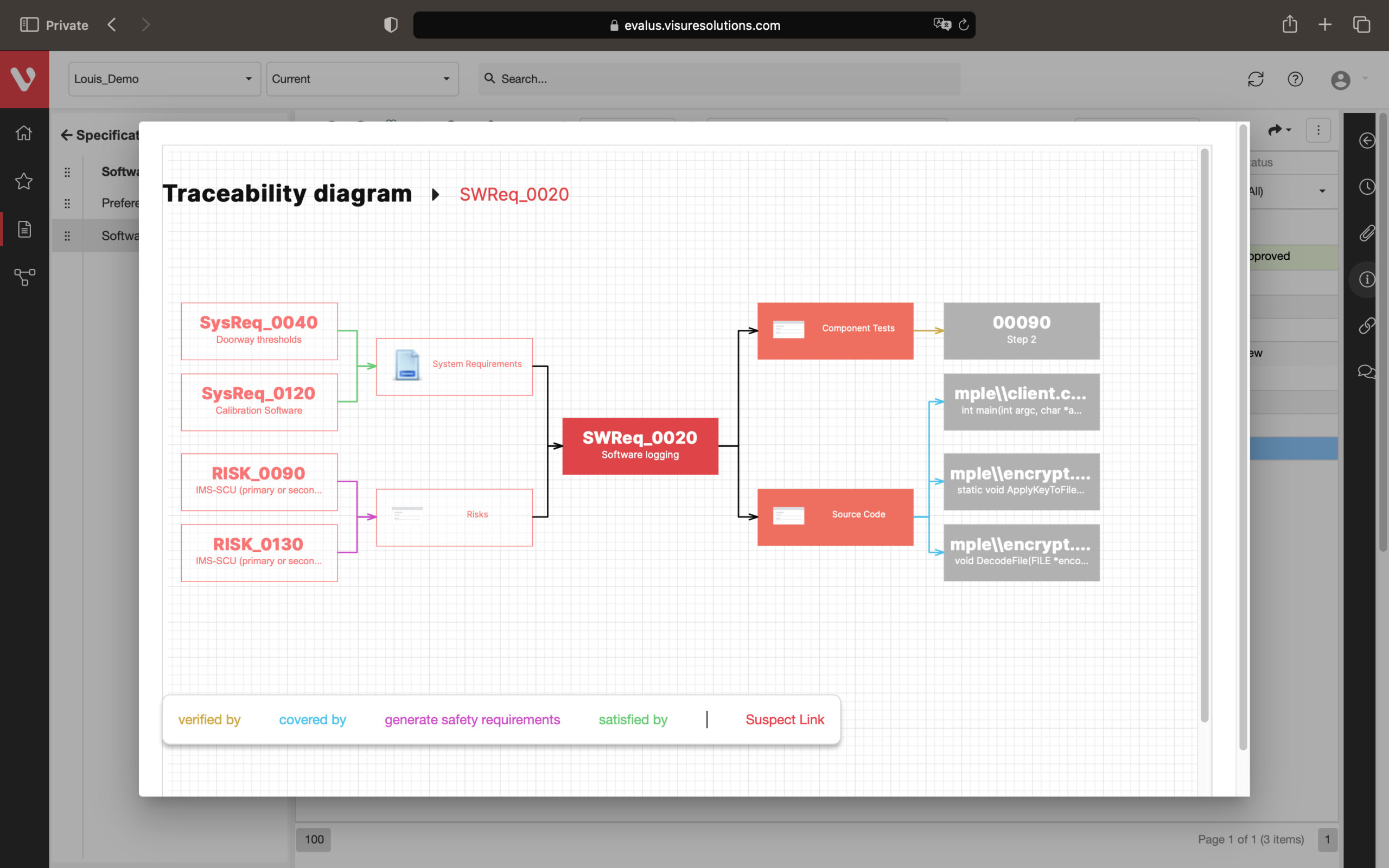
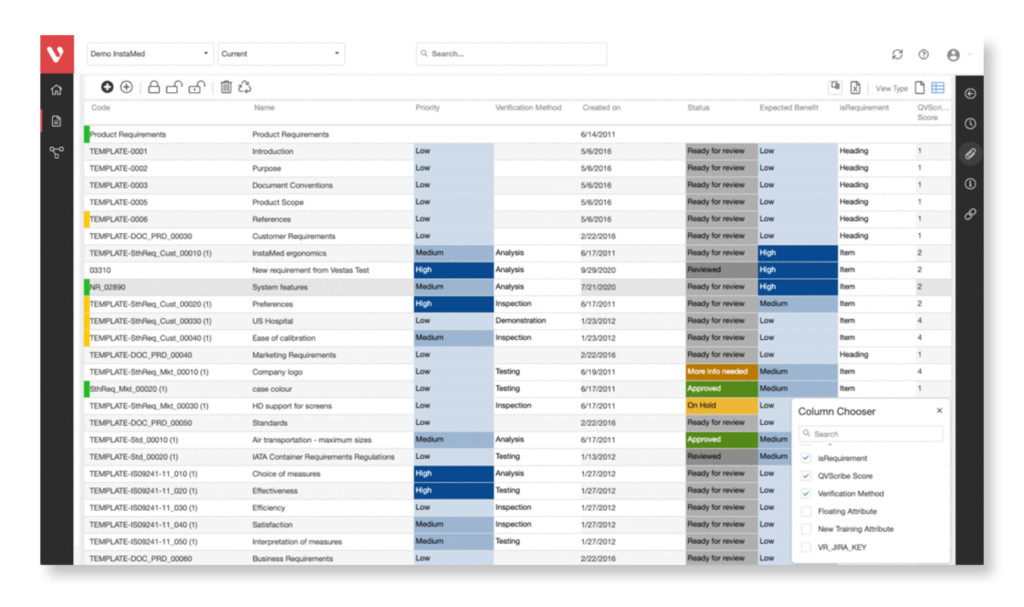
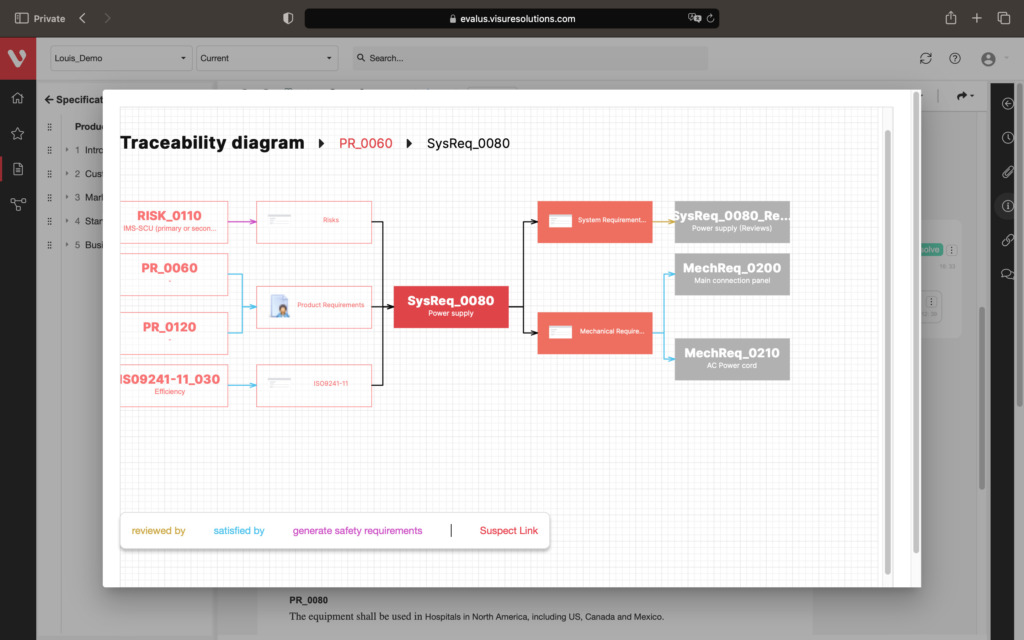
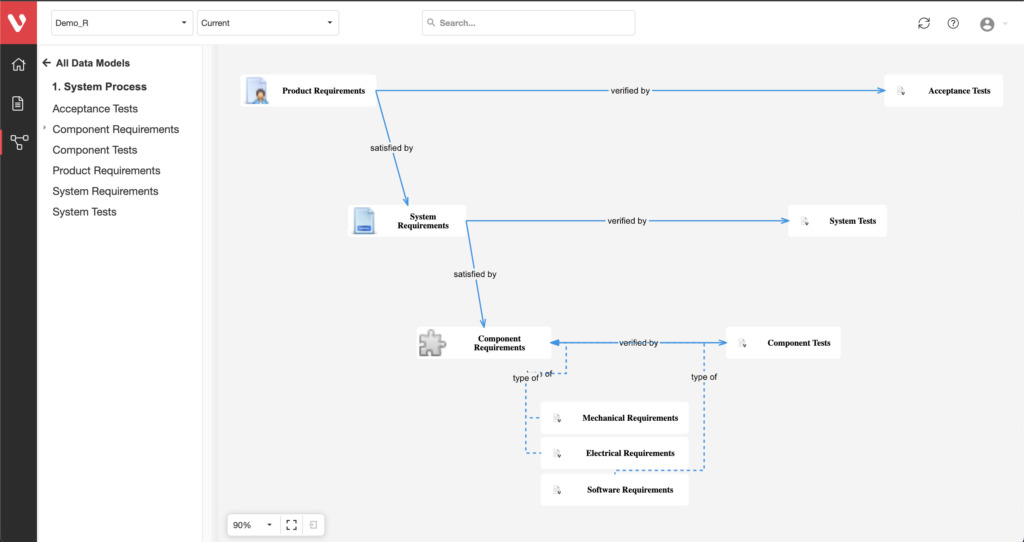
Effortlessly explore upstream and downstream relationships, anticipate change impact, monitor cross-project relationships, detect potential link issues during changes, and visualize relationship rules across projects to understand their organizational impact and reach.
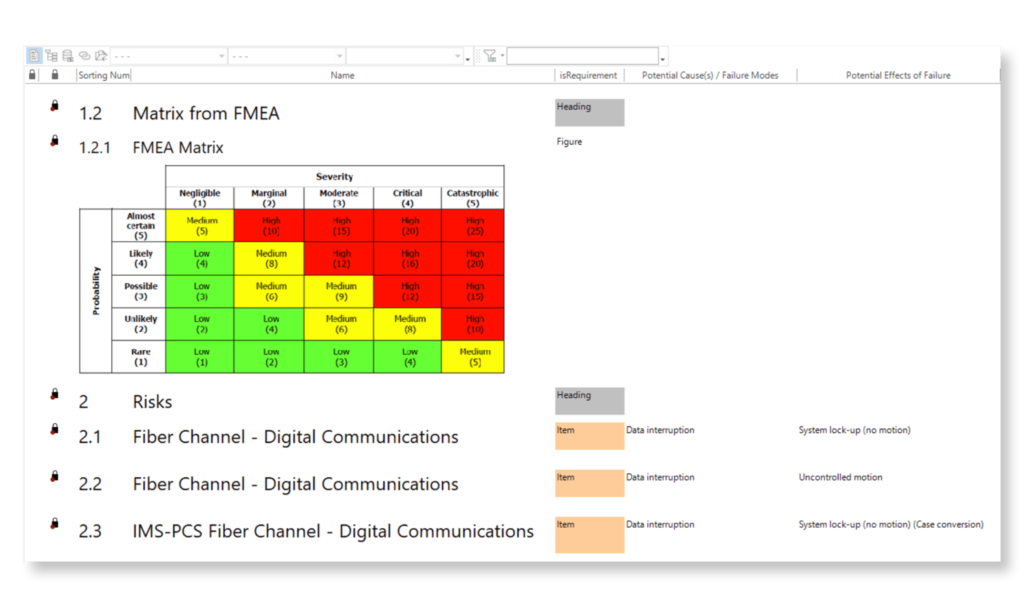
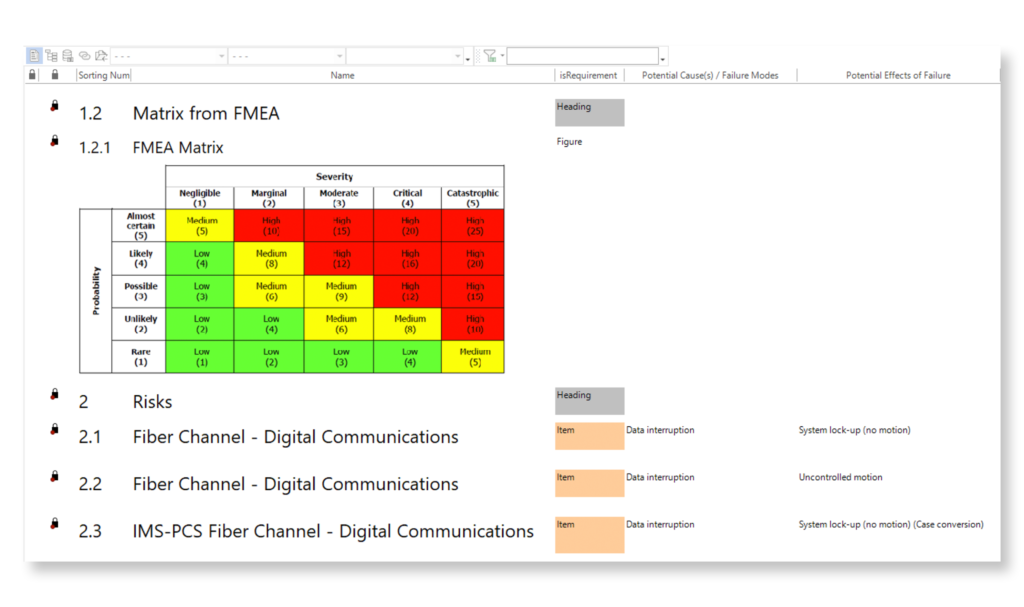
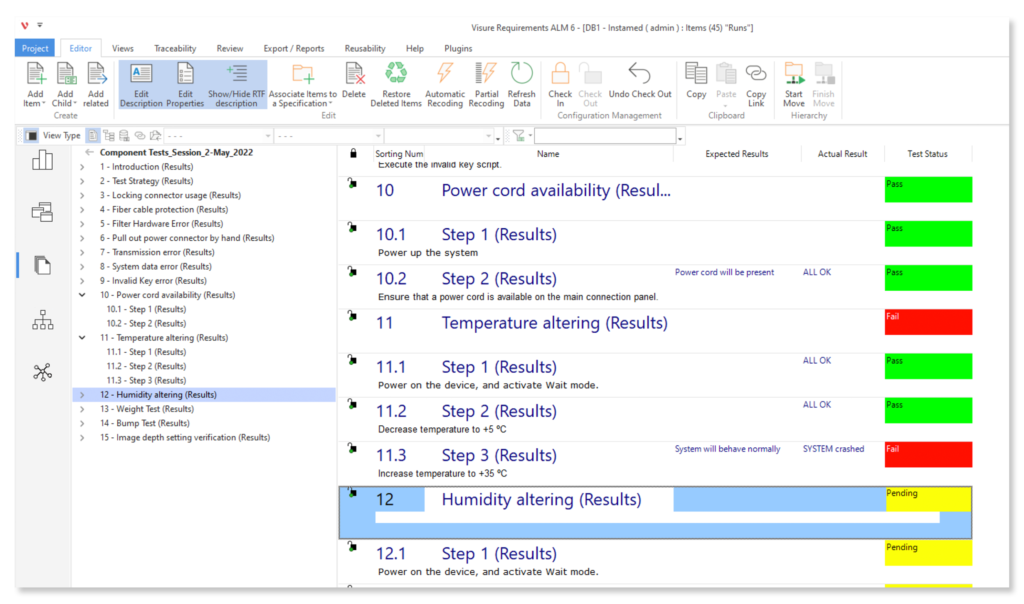
Keep your risk analysis current with real-time data, improve coverage by tracing open risks to requirements, build a comprehensive risk management profile using PHA and FMEA techniques, and guarantee quality and safety in intricate product development.
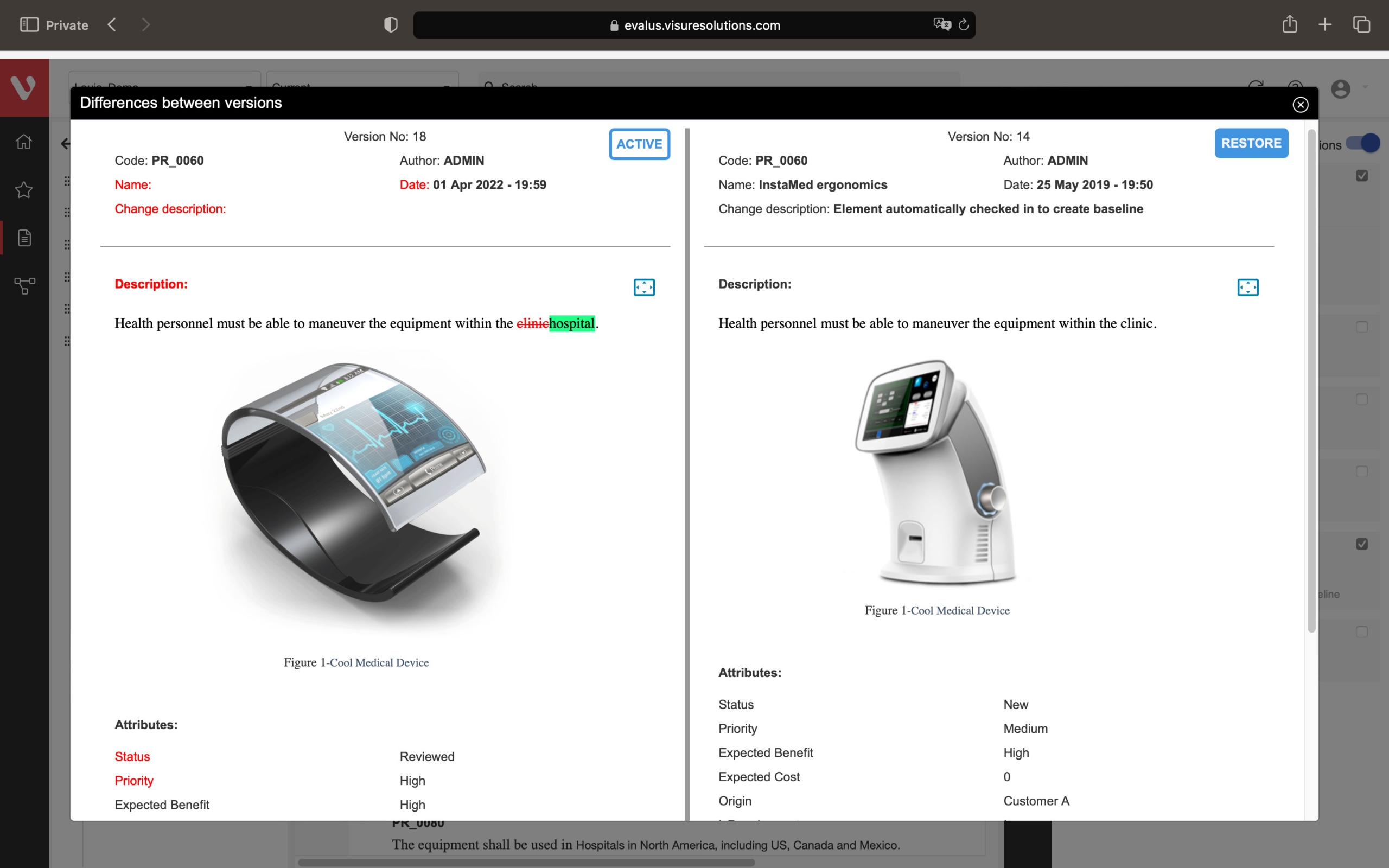
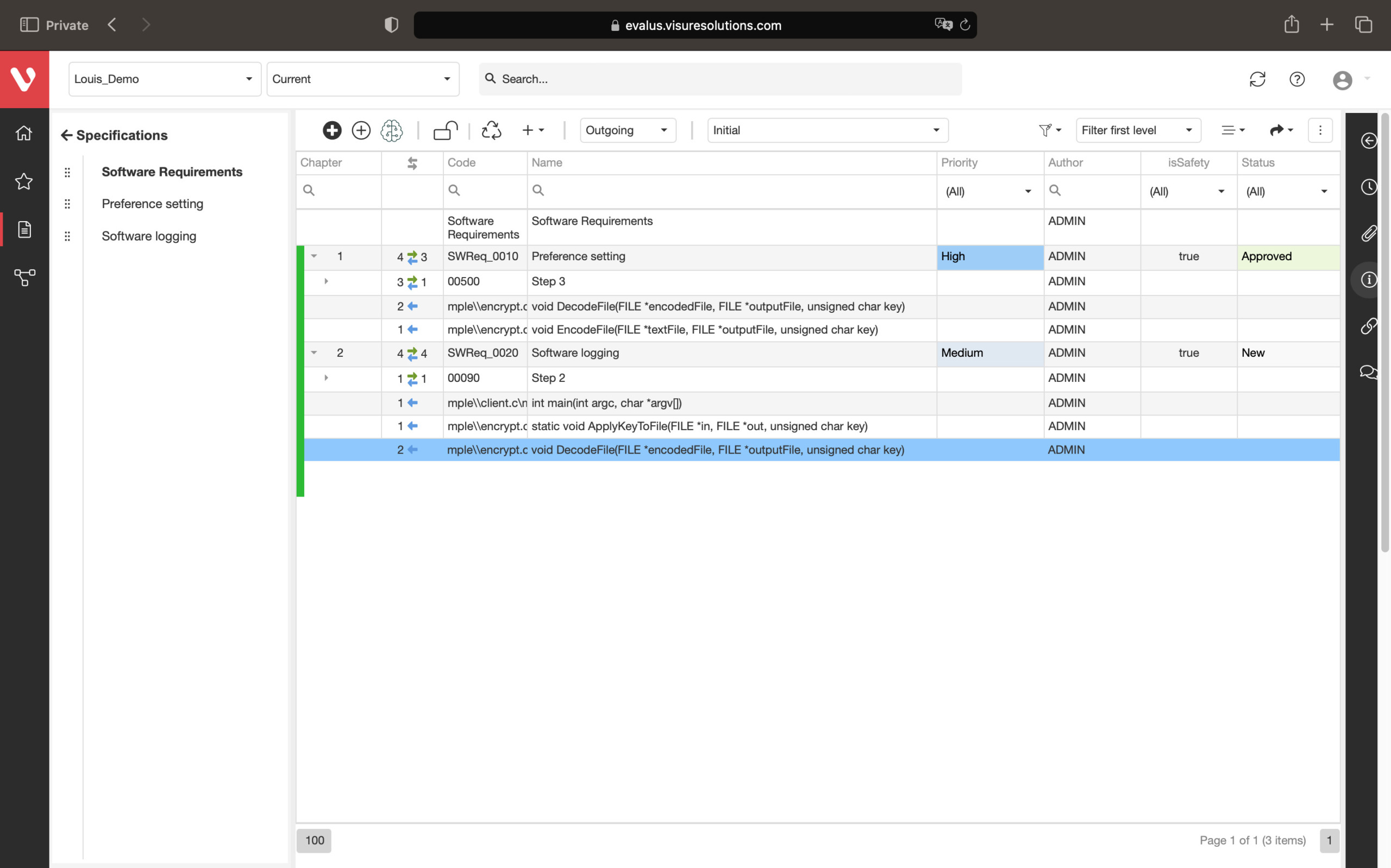
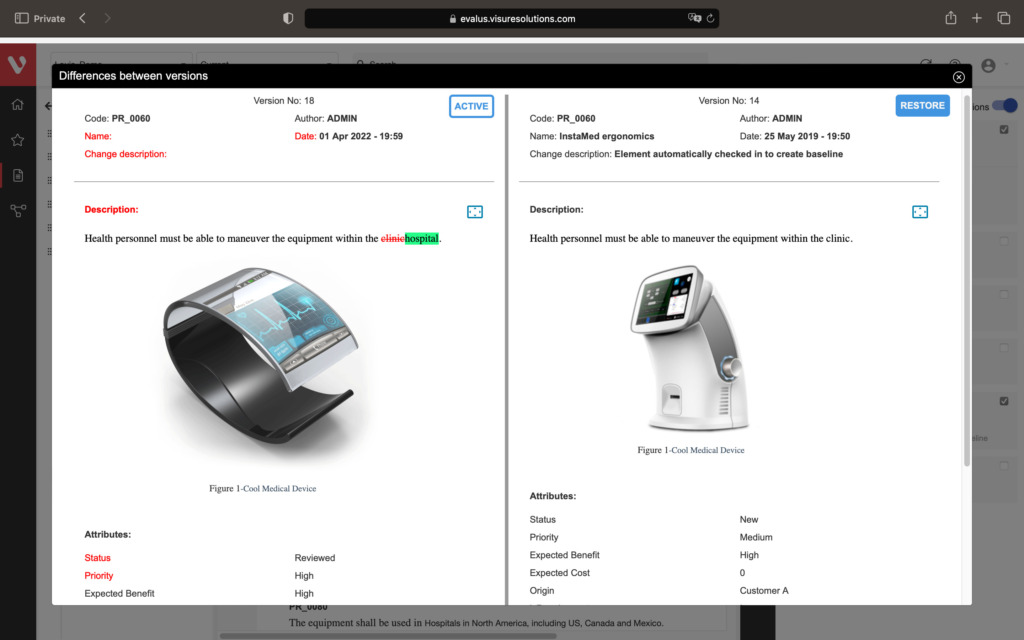
Speed up development and maintain consistency. Easily compare requirement versions, organize and secure your data, and create development stream baselines or requirement catalogs.
Compare changes in requirements, ensure data organization and security, snapshot project states, build reusable requirements catalogs, and develop product variants or new versions.
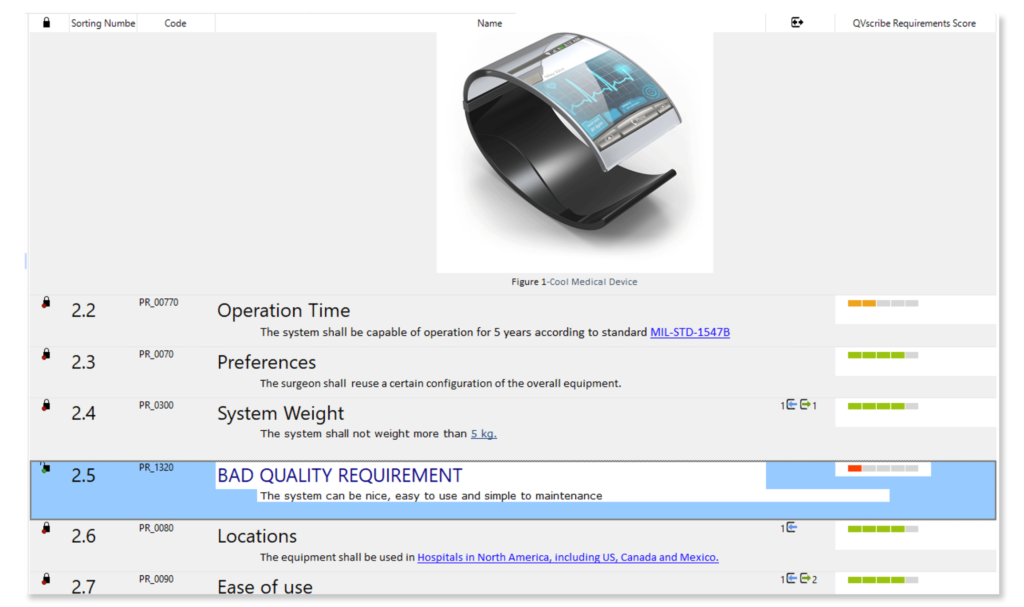
Automatically analyze the quality of requirements while writing them. Avoid ambiguous specifications from poorly written, ambiguous, and inconsistent requirements.

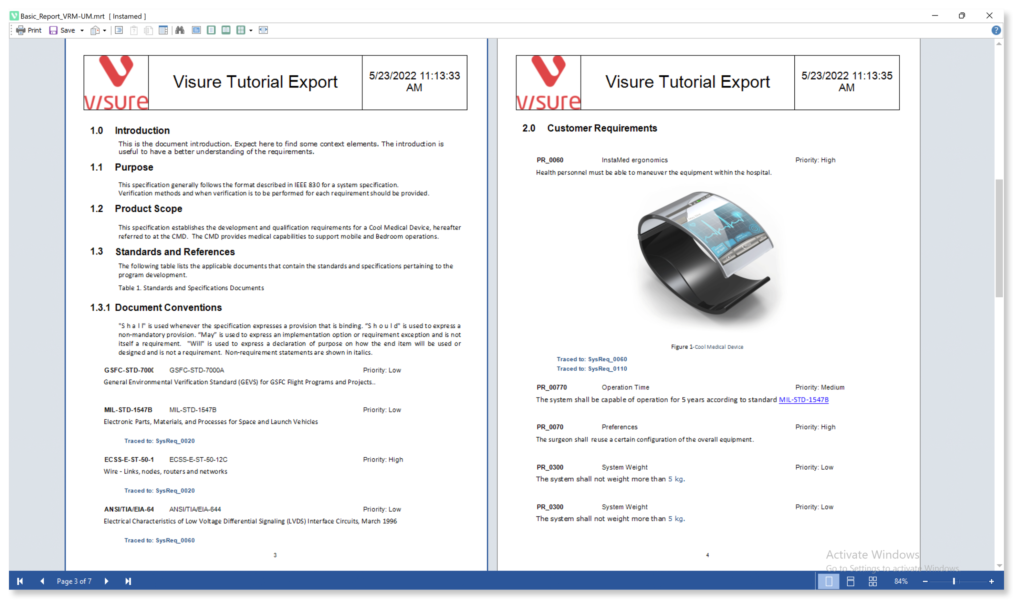
Increase your productivity and ease your Stakeholder review process by using simple import and export data features for ReqIF, IBM DOORS and MS Office Word & Excel.
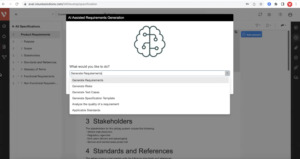
Leverage generative AI to analyze and optimize requirements writing and industry best practices, generate test cases and improve risk mitigation
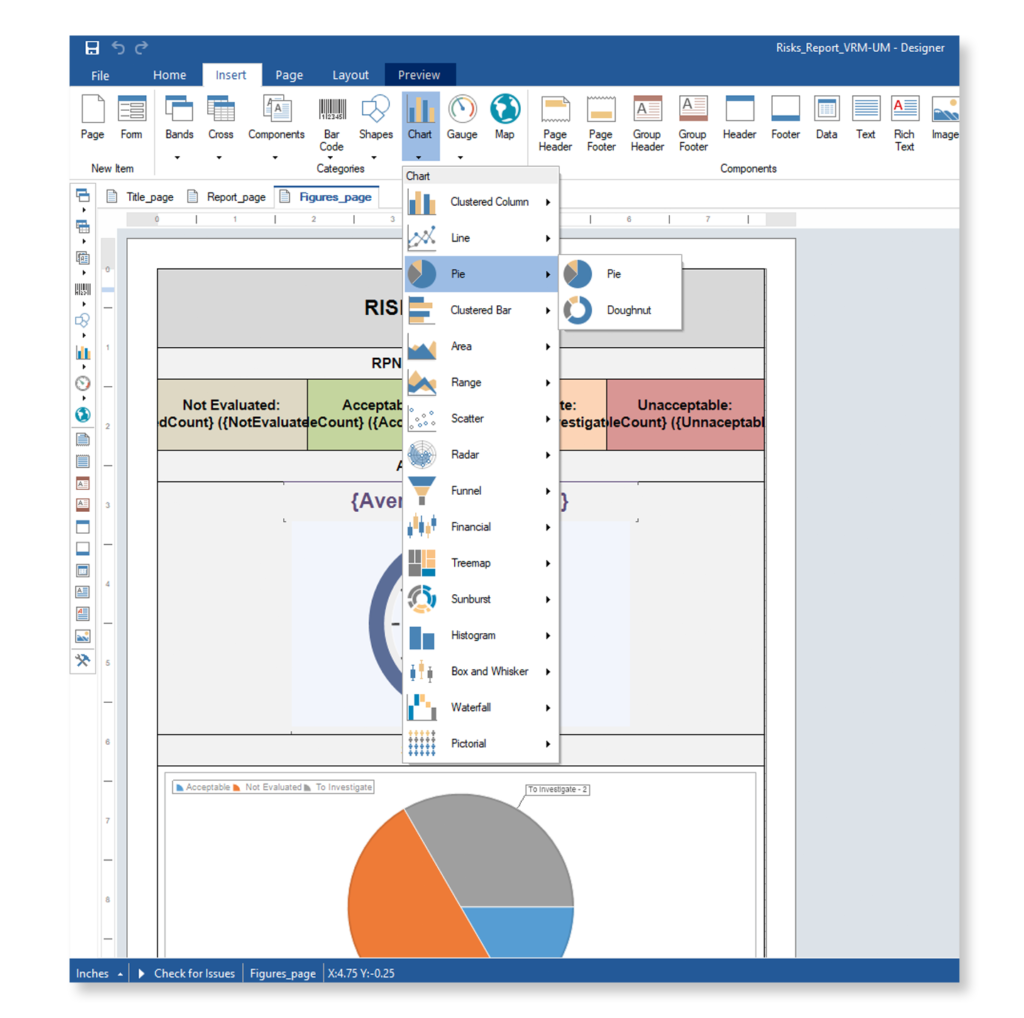
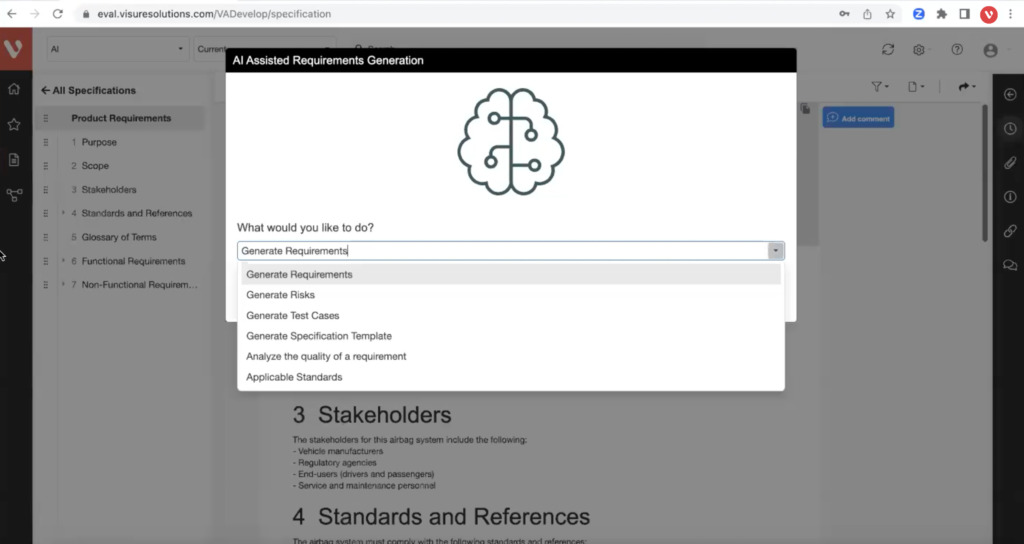
Why Top Leading Safety Critical Companies Manage Risks & FMEA with Visure
Visure Requirements Management Solution Connects with Best-of-Breed Tools
And even more integrations with other leading software — including automated test solutions— to accelerate and facilitate success across the entire product development lifecycle.
What industry professionals say about us





As posted in G2, SoftwareReviews and TrustRadius.
Ensure Regulatory Compliance.
Manage Risks & FMEA.
Accelerate Your Timelines.
- Most cost-effective
- Access All Features
- 30-Day Trial
On average, our customers experience:
See what’s possible with a Modern Risk Management and FMEA Software Solution
PER PROJECT
TO MARKET
PREPARING FOR AUDITS
The Ultimate Guide to Requirements Management & Traceability Best Practices
Hardware and software complexity is rapidly increasing across all highly regulated industries. Learn the best Requirements Management and Traceability practices that leading safety-critical companies use to mitigate risk and to manage product, systems, and software development.