#1 ALM SOFTWARE FOR MEDICAL DEVICES DEVELOPMENT
Enable Agile & Digital Engineering.
Enforce Traceability & Compliance.
Accelerate Your Timelines.
Simplify your complex product development, and begin ensuring end-to-end traceability and compliance across industry standards with Visure.
- Most cost-effective
- Access All Features
- 30-Day Trial


1,000+ Highly Regulated Organizations Trust Visure














Guarantee Medical Device Development Standards and Regulations Compliance with Industry Templates
Transform medical device development work across all design control, hardware and software engineering teams with a modern platform that is designed for medical device development safety-critical standards and regulations, resulting in speed time to market without sacrificing compliance, nor product quality.
- ISO 13485
- ISO 14971
- IEC 62304
- FDA 21 CFR 820.30
- FDA 21 CFR Part 11
- AAMI TIR45
- EU Regulations
- FMEA, FMECA, DFMEA

Achieve Project Success with an All-in-One AI-powered Medical Device ALM Software

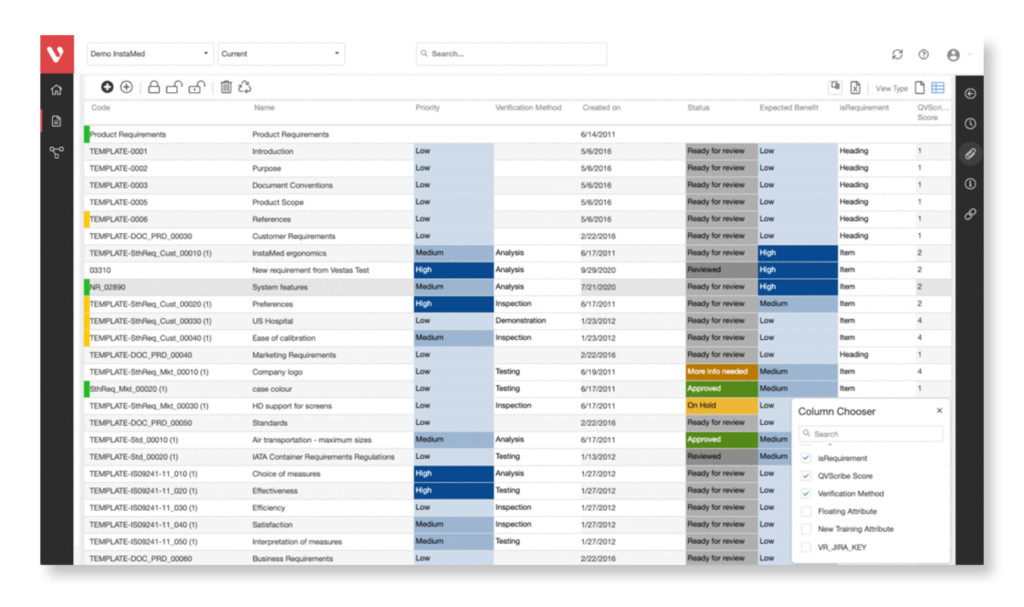
Achieve a balance between atomic requirements management and traceability, all while maintaining a familiar document-style approach.

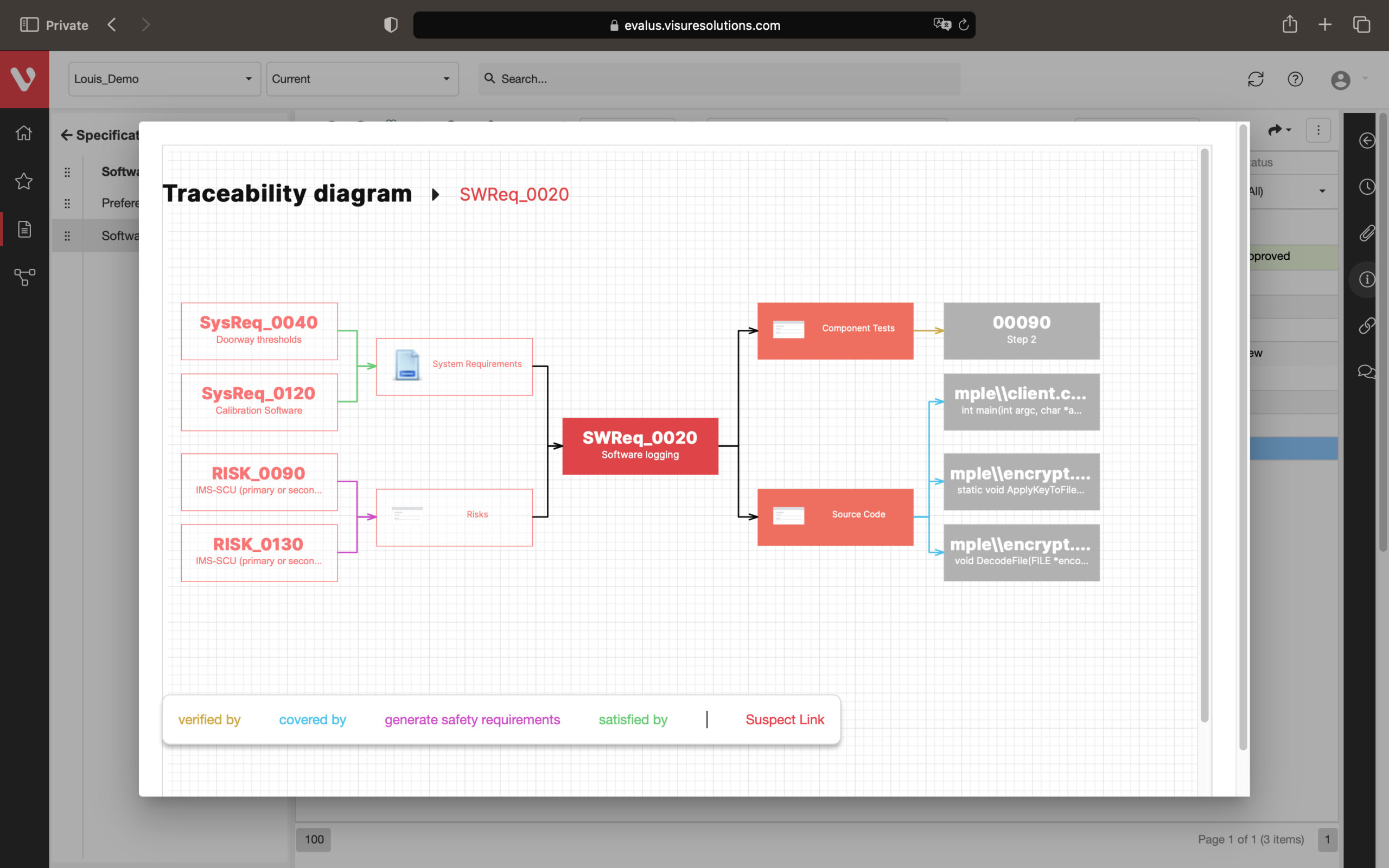
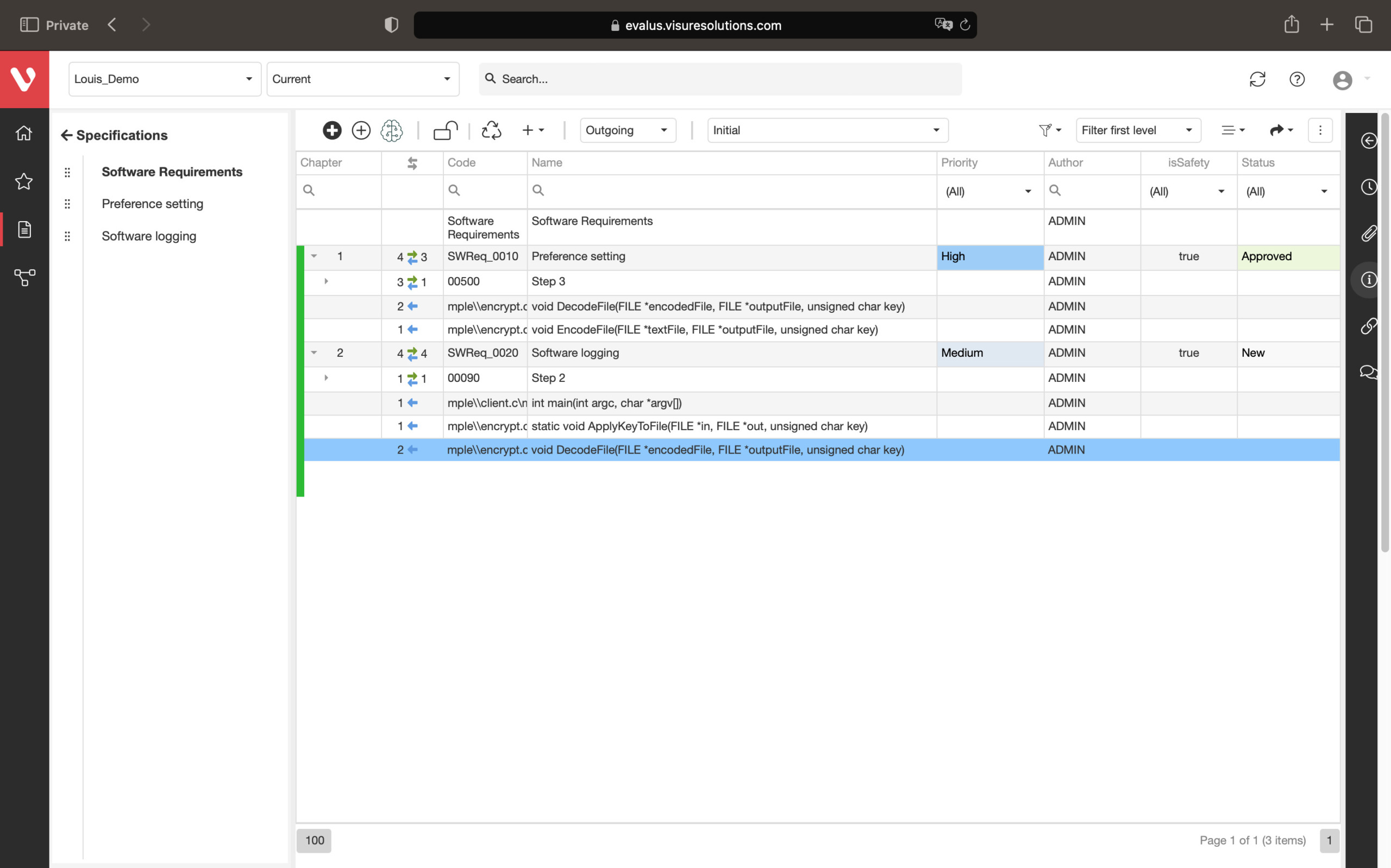
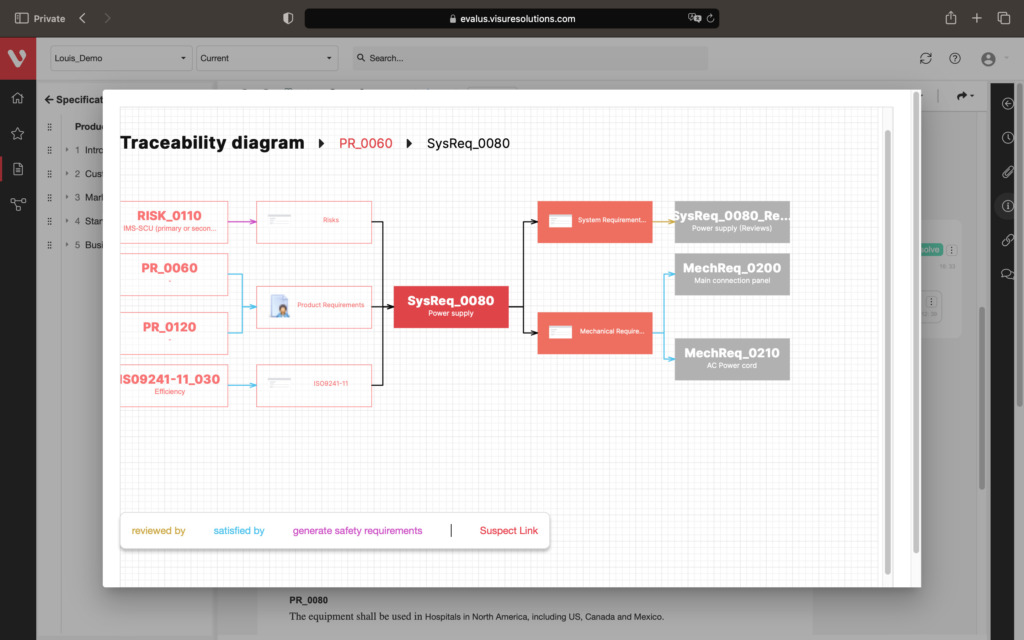
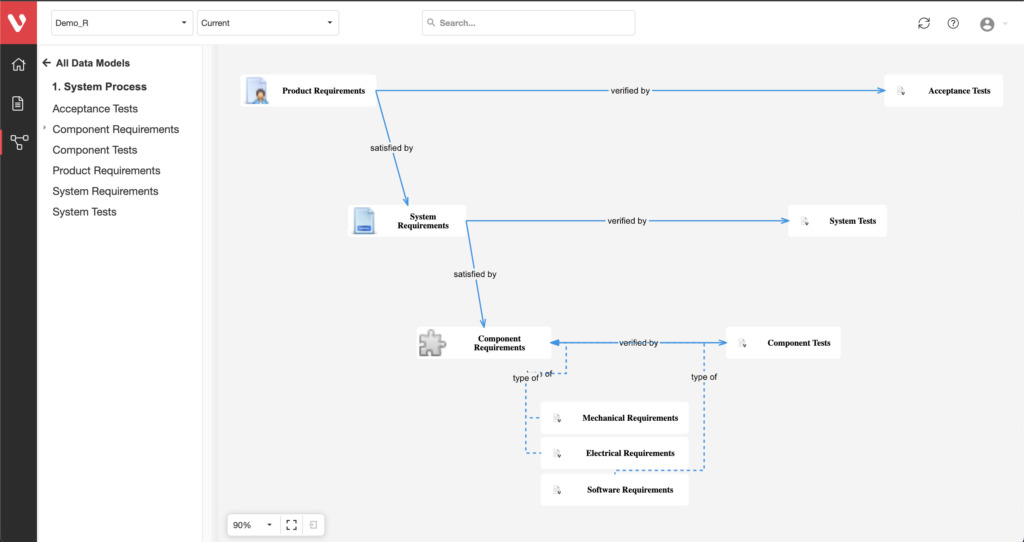
Effortlessly explore upstream and downstream relationships, anticipate change impact, monitor cross-project relationships, detect potential link issues during changes, and visualize relationship rules across projects to understand their organizational impact and reach.
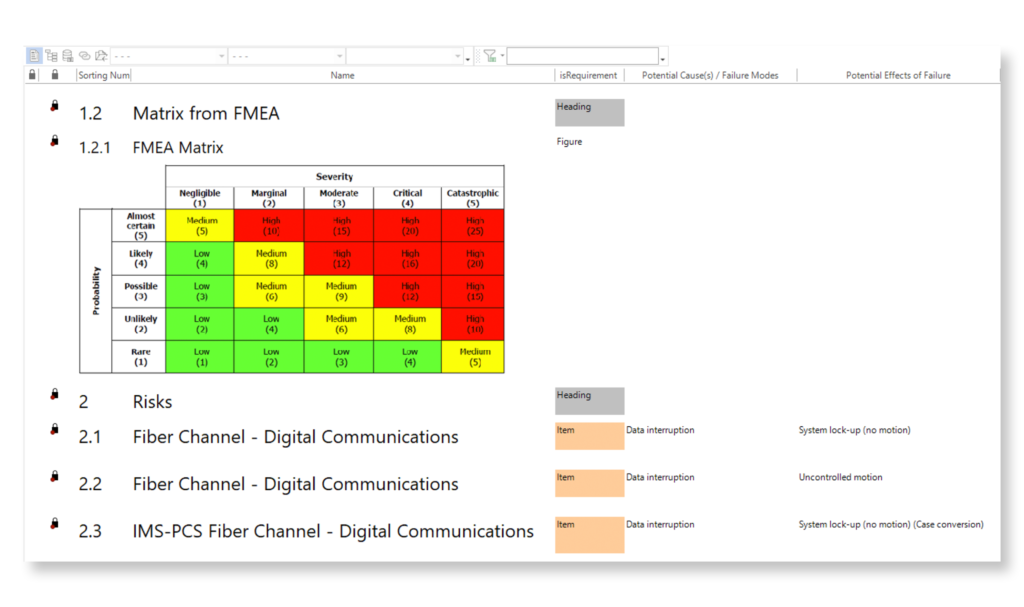
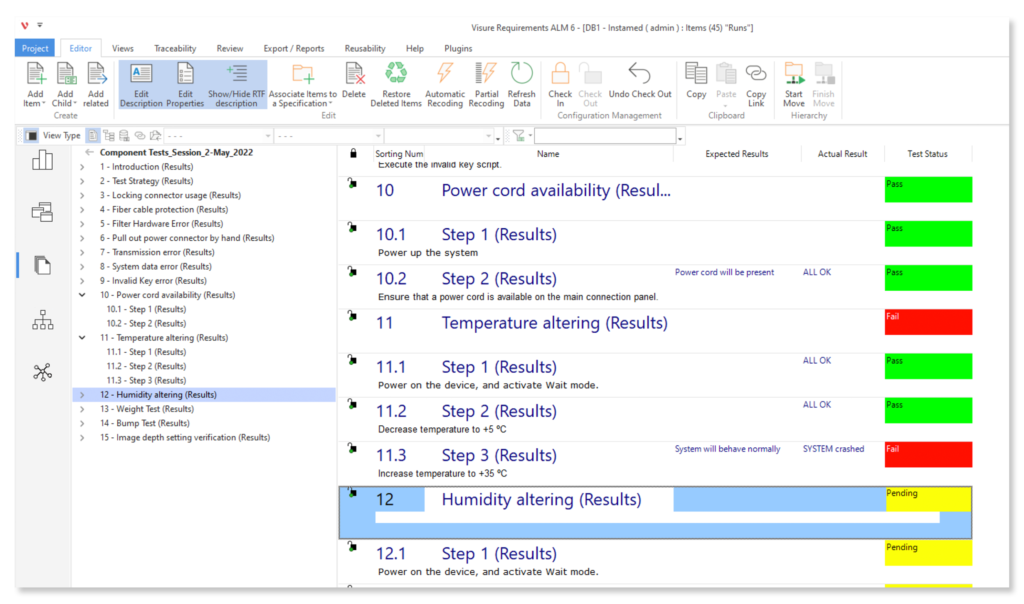
Keep your risk analysis current with real-time data, improve coverage by tracing open risks to requirements, build a comprehensive risk management profile using PHA and FMEA techniques, and guarantee quality and safety in intricate product development.
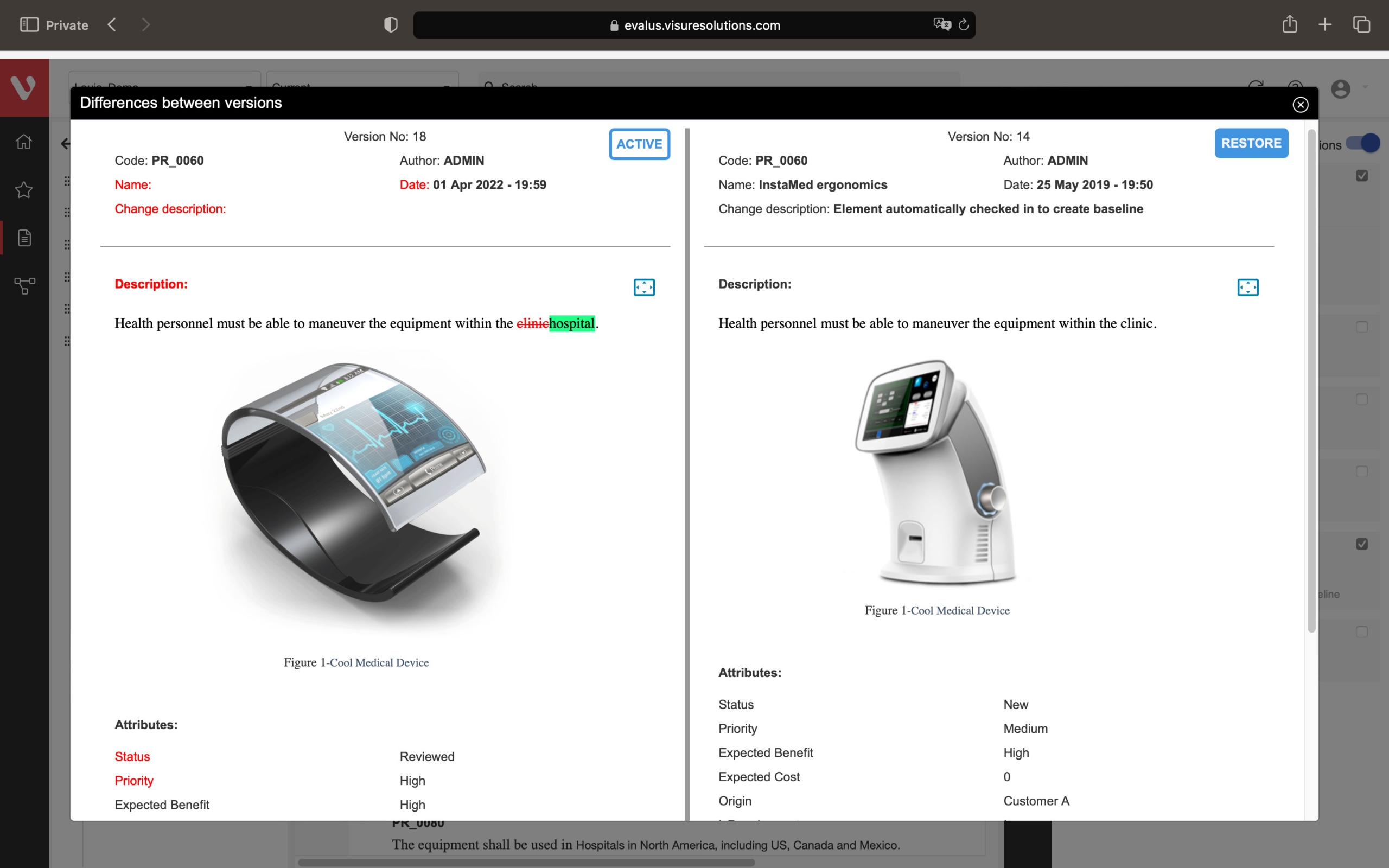
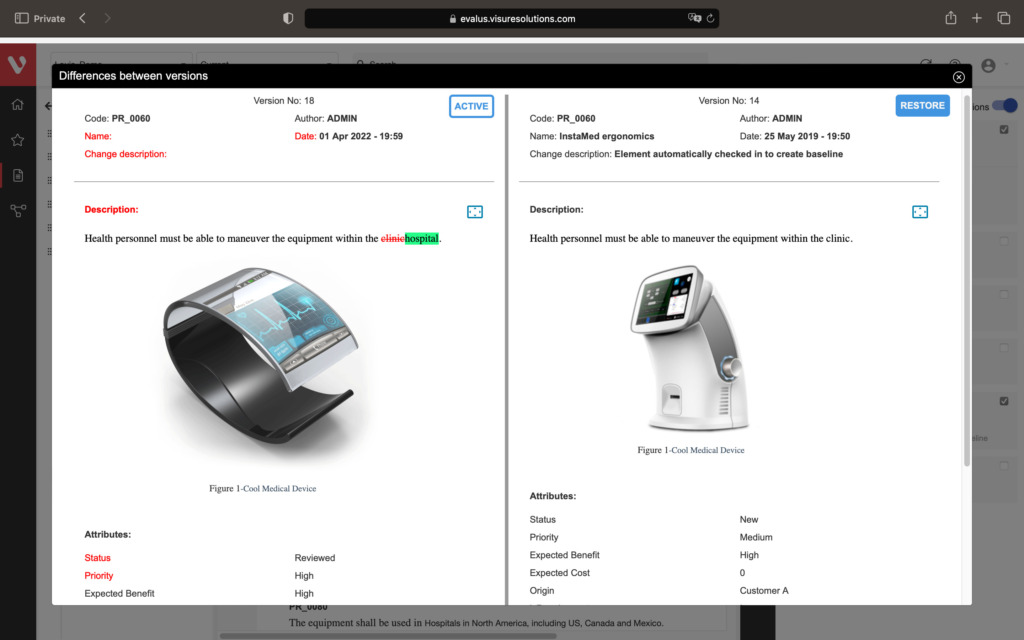
Speed up development and maintain consistency. Easily compare requirement versions, organize and secure your data, and create development stream baselines or requirement catalogs.
Compare changes in requirements, ensure data organization and security, snapshot project states, build reusable requirements catalogs, and develop product variants or new versions.
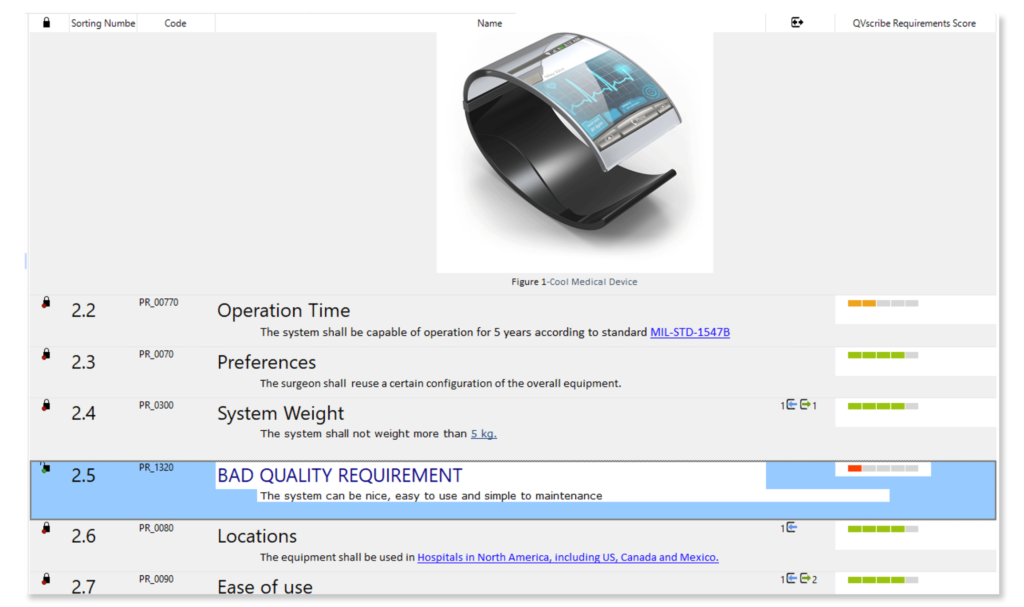
Automatically analyze the quality of requirements while writing them. Avoid ambiguous specifications from poorly written, ambiguous, and inconsistent requirements.

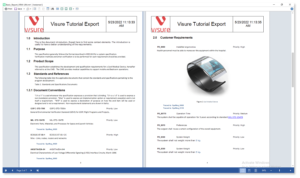
Increase your productivity and ease your Stakeholder review process by using simple import and export data features for ReqIF, and MS Office Word & Excel.
Automate Your Proof of Evidence.
Optimize Your DO-178C & DO-254 Certification Compliance.
With Visure, you can use automated checklists to manage compliance and easily integrate and access our DER partner’s checklists into our tool. This will enable you to design and improve a review process around these checklists.

Why Top Leading Medical Device Companies Choose Us
Visure Requirements ALM Connects with Best-of-Breed Tools
And even more integrations with other leading software — including automated test solutions— to accelerate and facilitate success across the entire product development lifecycle.
What industry professionals say about us





As posted in G2, SoftwareReviews and TrustRadius.
Ensure Medical Device Compliance.
Enforce Full Traceability.
Accelerate Your Timelines.
- Most cost-effective
- Access All Features
- 30-Day Trial
On average, our customers experience:
See what’s possible with a Modern Medical Device ALM Software Solution
PER PROJECT
TO MARKET
PREPARING FOR AUDITS
FAQs
What is included in Visure's Free trial?
During your free trial, you will have access to Visure’s key tool features, which includes: Requirement Management, Test management, Risk management, Baseline, Reusability, Version control, Review, Dashboard, Traceability Matrix, Impact Analysis Review, and Collaboration.
What is Visure’s Requirements ALM Pricing?
Great question! At Visure, we’ve been ranked as the most affordable modern requirements management tool, providing the most value to price solution in the market. Our pricing varies from company to company due to the different types of licenses and add-ons each one needs. Once we receive a couple of details regarding your needs, we will be able to provide you with an accurate pricing.
What types of licenses are available with Visure Requirements ALM?
We have two main types of licenses: Read & Write License and Read Only License. To Learn more about each type of license, please go over to our Pricing Page.
Do I need a credit card to get started?
No, neither credit cards nor contracts are required for your free trial.
You can test drive Visure, and cancel anytime.
Do I need to sign a contract to get started with my Visure free trial?
No, we don’t require any contracts to get started with Visure’s 30-day free trial.
What Add-ons are available for Visure Requirements ALM Platform?
- To complete the entire Application Development Lifecycle Management, we’ve developed Add-ons for Visure’s core Requirements ALM product. You can learn more about each add-on by visiting each of their pages. Our Add-ons include:
- Automated Checklists
- Report Manager
- Contributor
- Tool Qualification Package
- Quality Analyzer
- All Integrations
What integrations are available with Visure Requirements ALM Platform?
At Visure, we support and have native integrations with: VectorCAST, Jira, Azure DevOps, GitLab, Sparx EA, Word & Excel, and ReqIF. These native integrations are bi-directional, syncing the information automatically between tools. Your team can customize and configure the tool and its integration to fit your workflow methodologies, adapting to processes such a Waterfall, Agile, and Hybrid types of processes
In addition, with ReqIF, engineering teams can exchange data and documents across third party tools. These includes tools such as MATLAB Simulink, Cameo, Rapita Systems, IBM DOORS and Ansys Scade.
If we currently have another Requirements Management & ALM Tool, does Visure support migrations?
By working with 100+ global organizations, we learned that many are stuck with legacy tools and even tools that don’t completely satisfy their needs. For these reasons, at Visure we are committed on giving additional migration support and training when migrating to any of our tools.
Additionally, our ReqIF integration enables teams to easily migrate data between any other tool and Visure.
Where do I contact for Product Support?
If you’re an existing client and require product support, please visit our support page. In addition, if you’re currently evaluating Visure Requirements ALM Platform or any of our Add-on products, we’re committed on giving you any product, technical and customer support.
Does Visure offer tool trainings?
Great question! Yes, we do offer different levels of tool trainings.