Automate Your Proof of Compliance Process & Audits for FDA 21 CFR Part-11
Empower engineering teams building complex products or systems, while reducing project costs by simplifying your standard compliance process in a single platform.

Visure transforms the way these companies comply to industry standards and develop complex products.
What is FDA 21 CFR Part 11, and Why it's important?
FDA 21 CFR Part 11, titled "Electronic Records; Electronic Signatures", is of paramount importance within the pharmaceutical and medical device industries. Much like ISO 14971 in the medical device sector, FDA 21 CFR Part 11 places a significant emphasis on data integrity and security in electronic records and signatures.
This regulation is critical as it establishes stringent requirements for the use of electronic records and signatures in FDA-regulated industries. It ensures the reliability, authenticity, and integrity of electronic records, safeguarding data against tampering and ensuring its accuracy. Compliance with FDA 21 CFR Part 11 is essential to meet regulatory standards, maintain data integrity, and enhance the trustworthiness of electronic records and signatures, ultimately contributing to the safety and effectiveness of pharmaceuticals and medical devices.
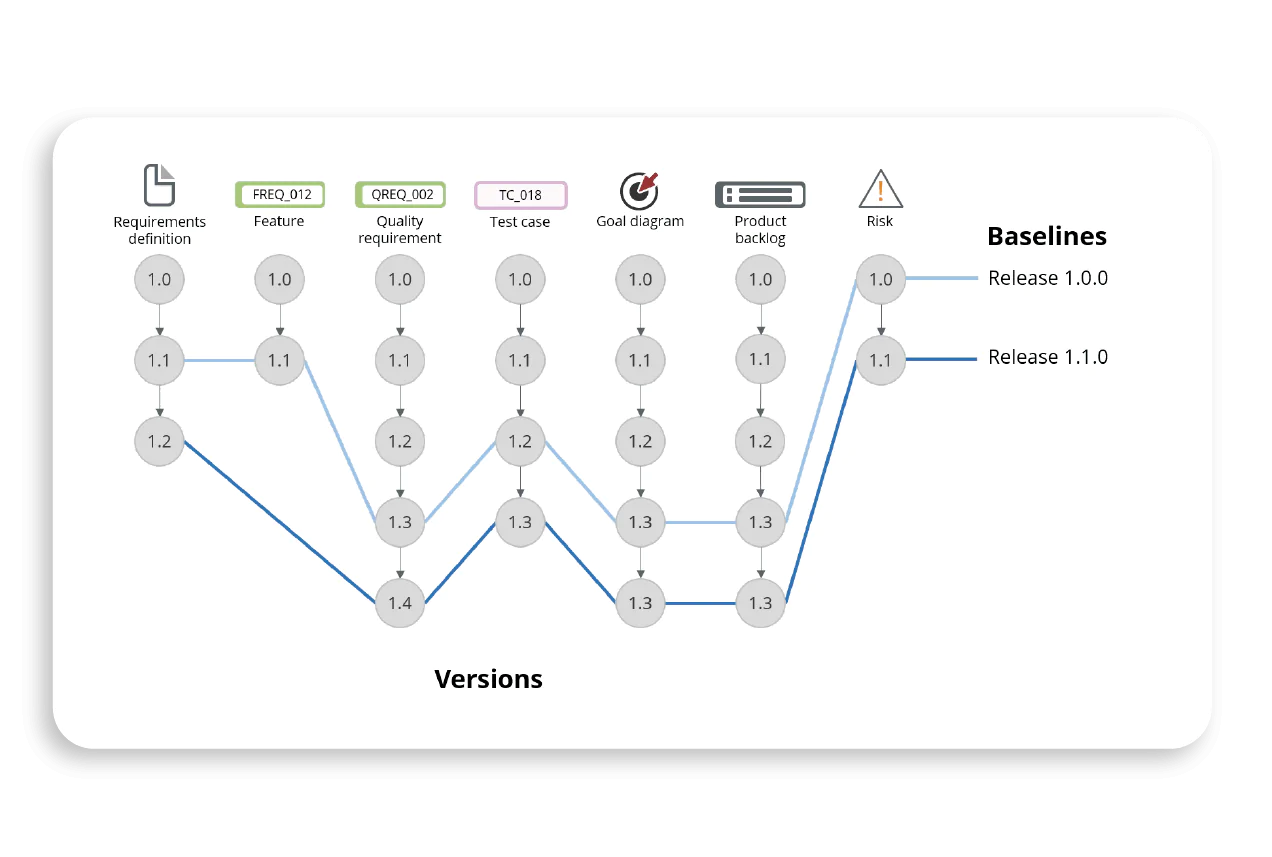
Tailor and Generate Baselines at Your Discertion With FDA 21 CFR Part 11 Complaince
Visure empowers you to generate baselines at any chosen moment. These baselines can encompass specific and precisely determined requirements, attributes, specifications, entire documents, or even entire projects.
Once an item is baselined, you gain unhindered access to it through various Visure tools, including Visure's Contributor, Client's Dashboard, and Authoring interfaces.
Moreover, your team possesses the capability to compare baselines across multiple projects, illuminating discrepancies between each one. This functionality seamlessly aligns with the stringent requirements and compliance standards set forth by FDA 21 CFR Part 11, ensuring data integrity, security, and accurate record-keeping in regulated industries such as pharmaceuticals and medical devices.
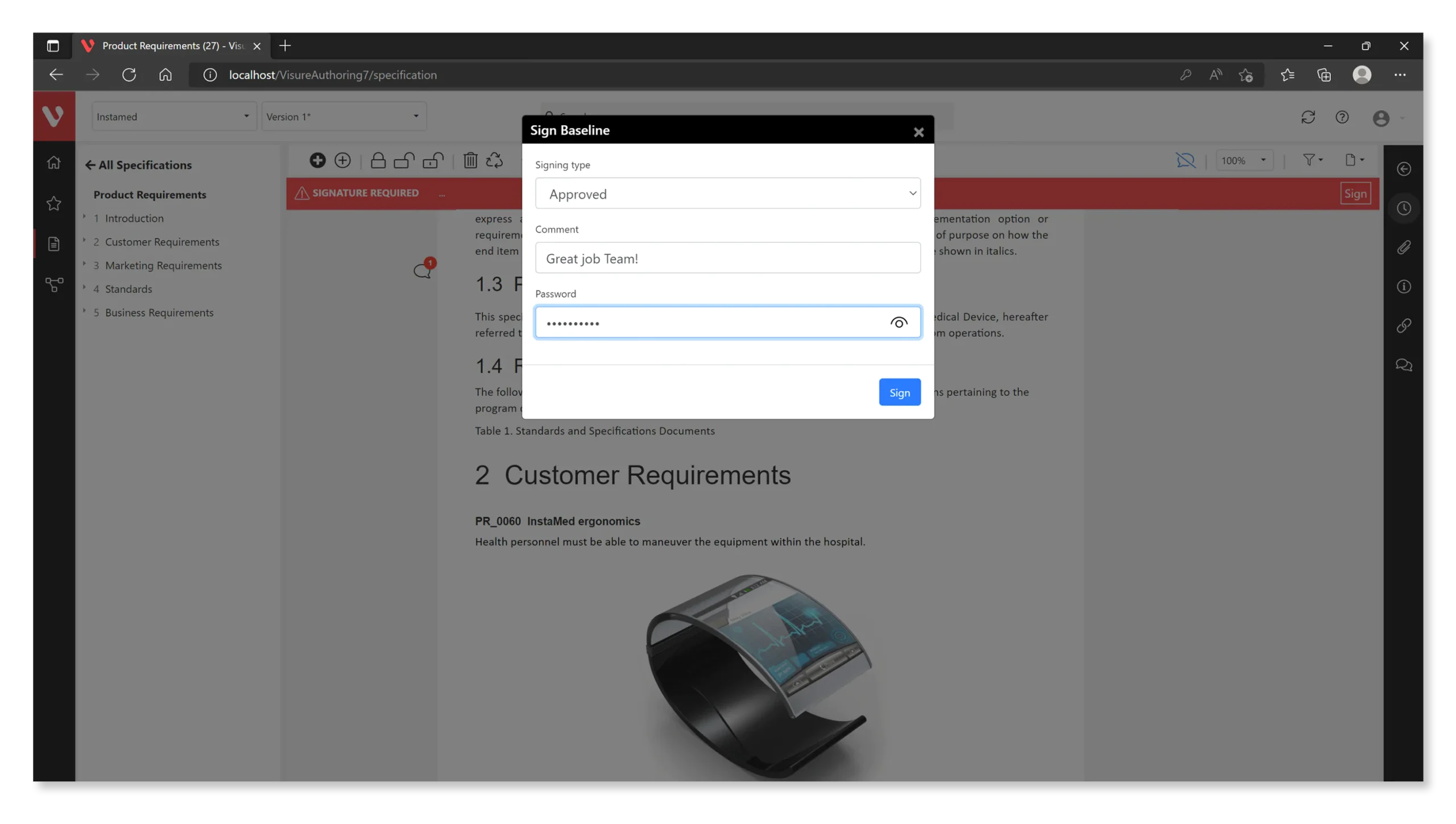
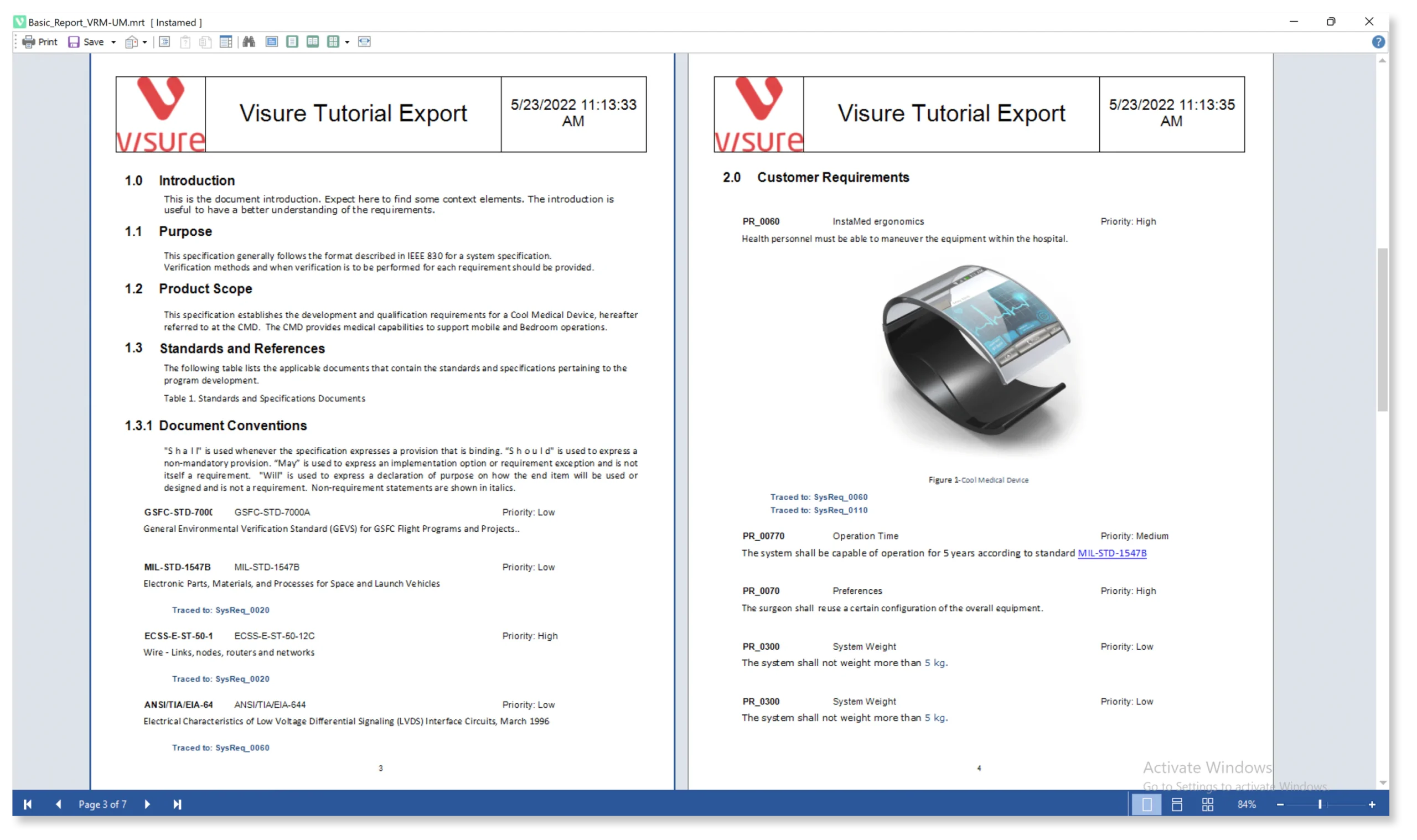
Streamline Your Baseline Signature Process With Automated Export in Multiple Formats
Visure offers the convenience of exporting your baseline process directly from the tool in various formats, including PDF, MS Office, and more. This flexibility empowers you to adhere to your preferred format for reviews and signatures, seamlessly integrating them into your documentation system for efficient and automated baseline management.
Why Top Leading Industry Organizations Choose Us

Reduce Risk & Manage Standard Compliance
Mitigate Risk and avoid stressful compliance audits across projects by centralizing and tracing in a single source of platform.
Full end-to-end Traceability, including Source Code
Configure your data model and gain full traceability between tests, requirements, risk, defects and all items, including source code to specific requirements.

Simple Import and Export Data
Increase your productivity by using simple import and export data features from ReqIF and MS Office Word and Excel.

Facilitate Real Time Collaboration & Alignment
Visure integrates bi-directionally and automatically with the top industry engineering tools, easing collaboration among teams in real time.

Easy to use UX/UI Requirements ALM Tool
Forget about legacy tools user friendly experience, and implement an easy to use Requirements ALM tool with a low learning curve.

Most Value to Price Product in the Market Guaranteed
We are committed to your team's project success by delivering within budget. That's why Visure's pricing is a fraction from other competitors.
Maintain Security Across Development
With our On-Premise Licensing option, you can easily deploy and maintain security across all your projects within the tool.

Accelerate Project Speed to Market
Increase your team's productivity with reusability of components across projects and automating repetitive tasks through open source code & AI.

Access Premium Support, Trainings and Consultations
Fast track your team's success by getting your team up and running easily, while staying on top with industry best practices.
Integrate Seamlessly with Top Tier Engineering Tools
Start Automating Your Compliance Through End-to-End Traceability with Visure Today.
Start 30-day free trial