Accelerate Innovation & Compliance in Pharma and Biomedical Product Development
Simplify your complex pharmaceutics and biomedical product development, and begin ensuring end-to-end traceability and compliance across industry standards with Visure.
Visure transforms the way these companies develop products and complete projects.
What is GAMP5? How is it important?
GAMP 5, short for "Good Automated Manufacturing Practice 5," is a vital framework within the pharmaceutical and biopharmaceutical industries. GAMP 5 emphasizes the importance of risk management and validation in the development and use of automated systems.
This framework is indispensable as it provides a structured approach to computerized systems validation and risk management, ensuring the reliability and integrity of automated processes. GAMP 5 sets forth comprehensive guidelines for the lifecycle management of automated systems, covering aspects like risk assessment, testing, and documentation. Compliance with GAMP 5 is essential for pharmaceutical and biopharmaceutical companies to meet regulatory requirements, guarantee product quality, and enhance patient safety, ultimately contributing to the integrity and effectiveness of automated systems in these industries.
Ensure Adherence to Pharmaceutical product Development Standards and Regulations With Specialized Industry Templates
Revolutionize pharmaceutical product development processes across all engineering teams by leveraging a cutting-edge platform meticulously crafted to align with safety-critical standards and regulations, as guided by GAMP 5.
This transformation leads to expedited time-to-market and reduced validation expenses, all while maintaining unwavering compliance and product quality standards.
Integrate with Top Industry Solutions, Migrate from Legacy Tools, and Import & Export from and to MS Office easily.
Most global pharma companies come from legacy old tools, such as IBM Doors, that are slowing them down, while also unable them to digitalize their engineering teams. For this reason, at Visure we created easy import and export features from IBM DOORs as well as a simple migration feature to get started.
Additionally, with Visure you can access the best import and export feature from MS Office Word & Excel, and ease collaboration across supply chain by using international standard ReqIF for Data Exchange.
By gaining access to these features and top tier industry solutions integrations, you avoid manual rework through roundtrip requirements over multiple interactions, loss free, and without duplication.
Now you verify that all requirements are met, regardless of their original source, and in one single platform.

Why Top Leading Industry Organizations Choose Us

Reduce Risk & Manage Standard Compliance
Mitigate Risk and avoid stressful compliance audits across projects by centralizing and tracing in a single source of platform.
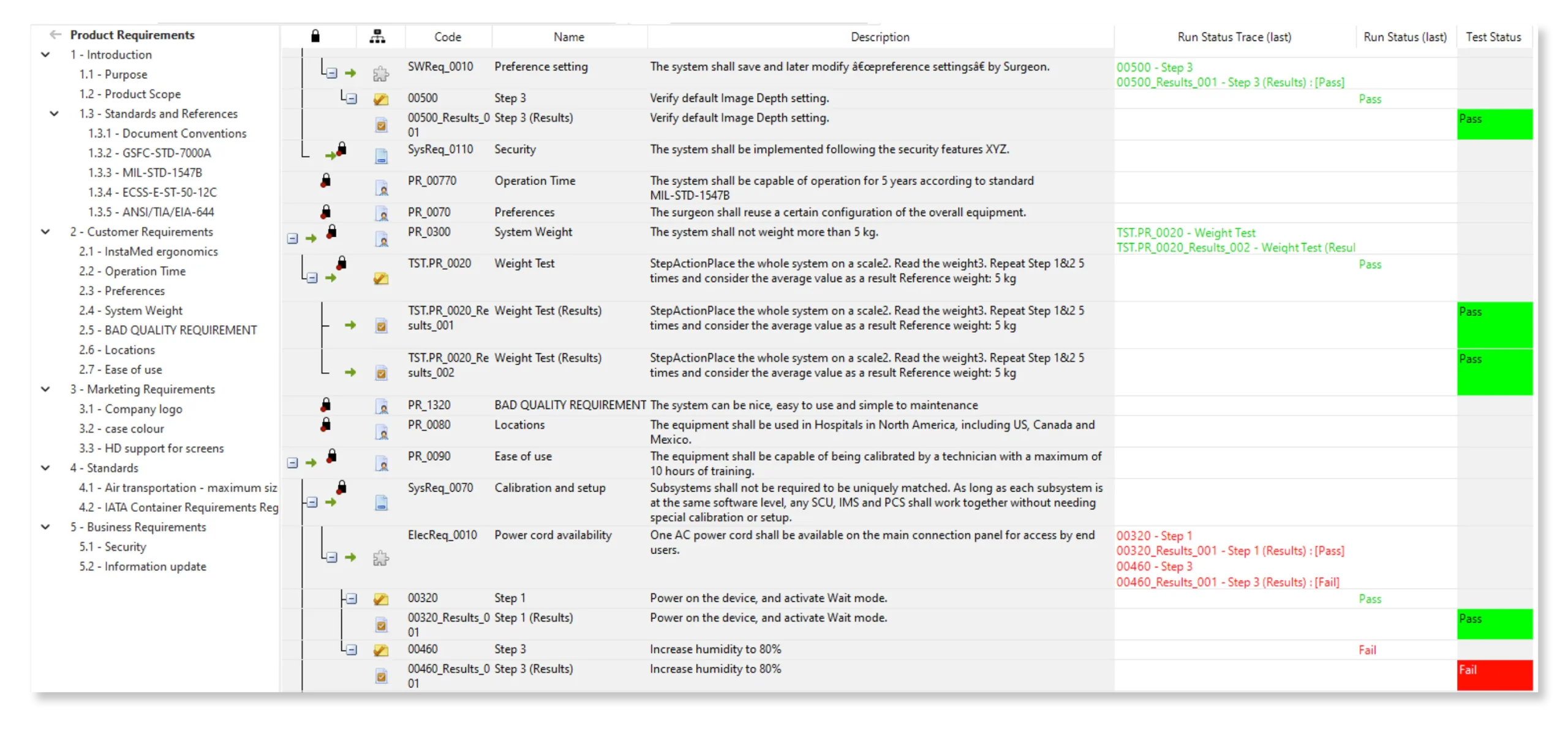
Full end-to-end Traceability, including Source Code
Configure your data model and gain full traceability between tests, requirements, risk, defects and all items, including source code to specific requirements.

Simple Import and Export Data
Increase your productivity by using simple import and export data features from ReqIF and MS Office Word and Excel.

Facilitate Real Time Collaboration & Alignment
Visure integrates bi-directionally and automatically with the top industry engineering tools, easing collaboration among teams in real time.

Easy to use UX/UI Requirements ALM Tool
Forget about legacy tools user friendly experience, and implement an easy to use Requirements ALM tool with a low learning curve.

Most Value to Price Product in the Market Guaranteed
We are committed to your team's project success by delivering within budget. That's why Visure's pricing is a fraction from other competitors.
Maintain Security Across Development
With our On-Premise Licensing option, you can easily deploy and maintain security across all your projects within the tool.

Accelerate Project Speed to Market
Increase your team's productivity with reusability of components across projects and automating repetitive tasks through open source code & AI.

Access Premium Support, Trainings and Consultations
Fast track your team's success by getting your team up and running easily, while staying on top with industry best practices.
Visure Connects with Best-of-Breed Tools in the Pharmaceutical Industry
Simplify your Complex Pharma Product Development with Visure's Modern Requirements ALM Platform today.
Start 30-Day Free trial