#1 IEC 62304 SOFTWARE & ALM TOOL
Ensure IEC 62304 Compliance.
Enforce Traceability.
Ease & Accelerate Your Audits.
Unlock efficiency and empower engineering teams with the leading IEC 62304 & ALM software tailored to Medical Device companies and designed to enforce traceability, and improve speed and quality while lowering risk and cost.
- Most cost-effective
- Access All Features
- 30-Day Trial


1,000+ Highly Regulated Organizations Trust Visure















What is IEC 62304, and Why is it important?
Functional safety is an important consideration in the design and maintenance of medical device software. IEC 62304 is a functional safety standard that covers the safe design and maintenance of software. It provides processes, activities, and tasks to ensure safety.
The standard covers a wide range of aspects related to functional safety, including hazard analysis and risk management, software development and testing, configuration management, and change control. By following the processes and tasks outlined in IEC 62304, developers can help ensure the safe design and operation of their software.
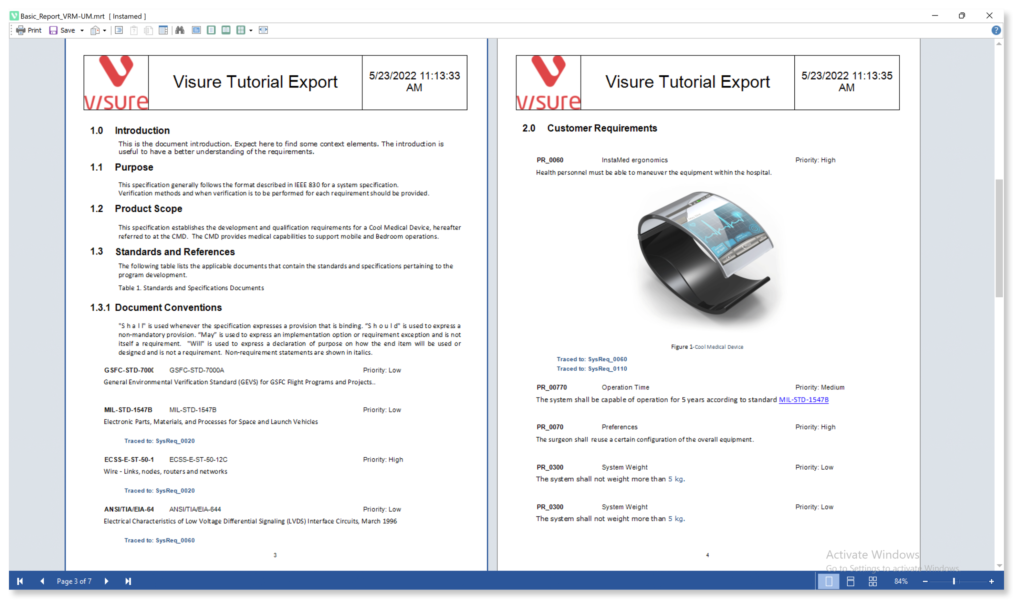
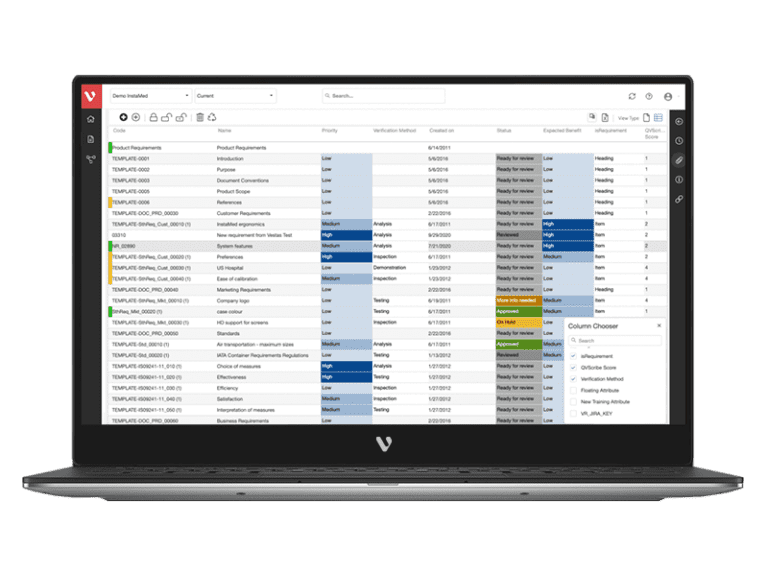
Guarantee Medical Device Standards and Regulations Compliance with Industry Templates
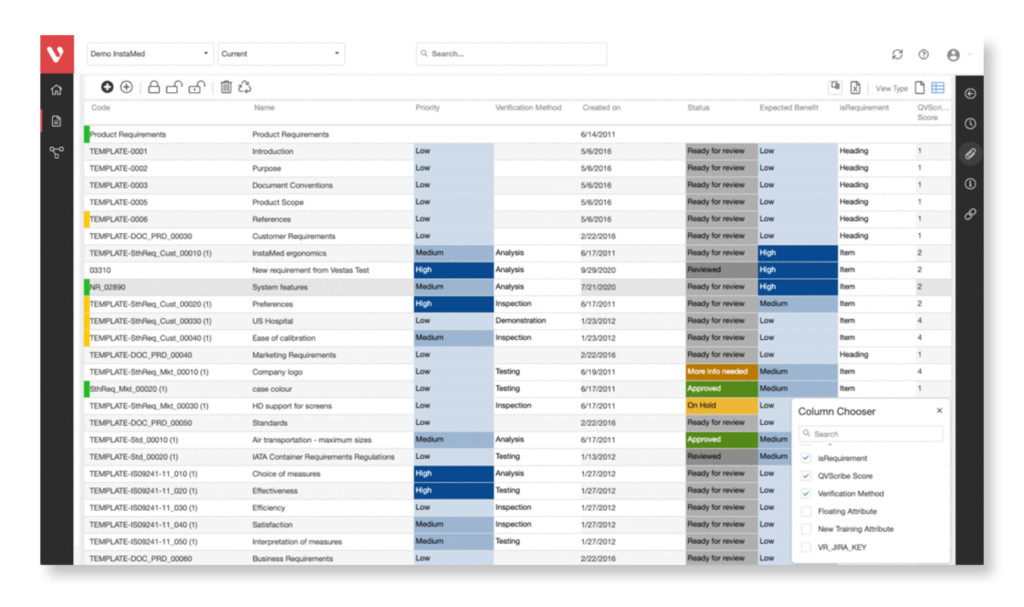
Visure Requirements ALM centralizes requirements, tests, defects and risks in a single platform, gaining control and a full end-to-end traceability. This helps organizations in enforcing their process to comply with IEC 62304 and creating the necessary deliverables to meet the software safety classes.
- End-to-End Traceability
- Increase Productivity & Alignment
- Product Quality with Dedicated Templates
- Saves Time, Money, & Resources
- Customisable Reports & Metrics
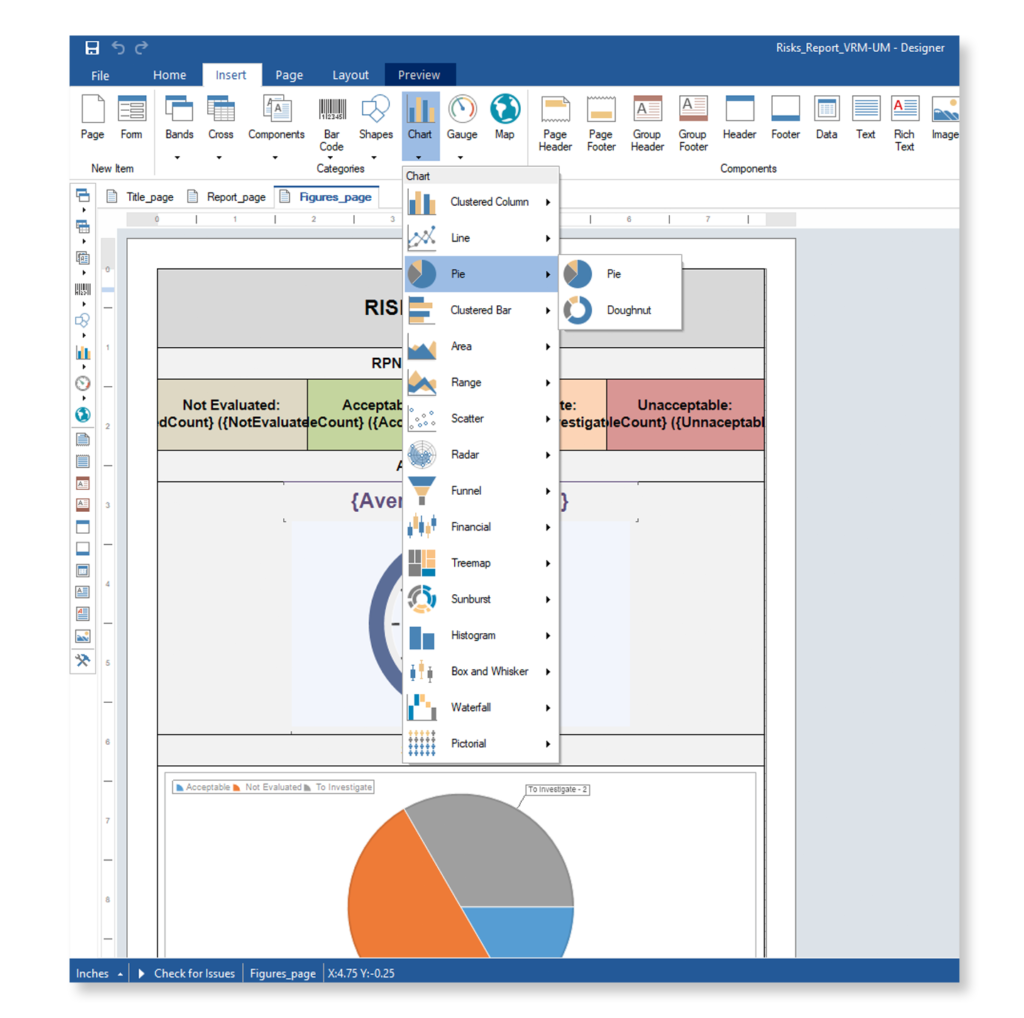
Achieve IEC-62304 Compliance with an All-in-One AI-powered Medical Device ALM Software

Achieve a balance between atomic requirements management and traceability, all while maintaining a familiar document-style approach.

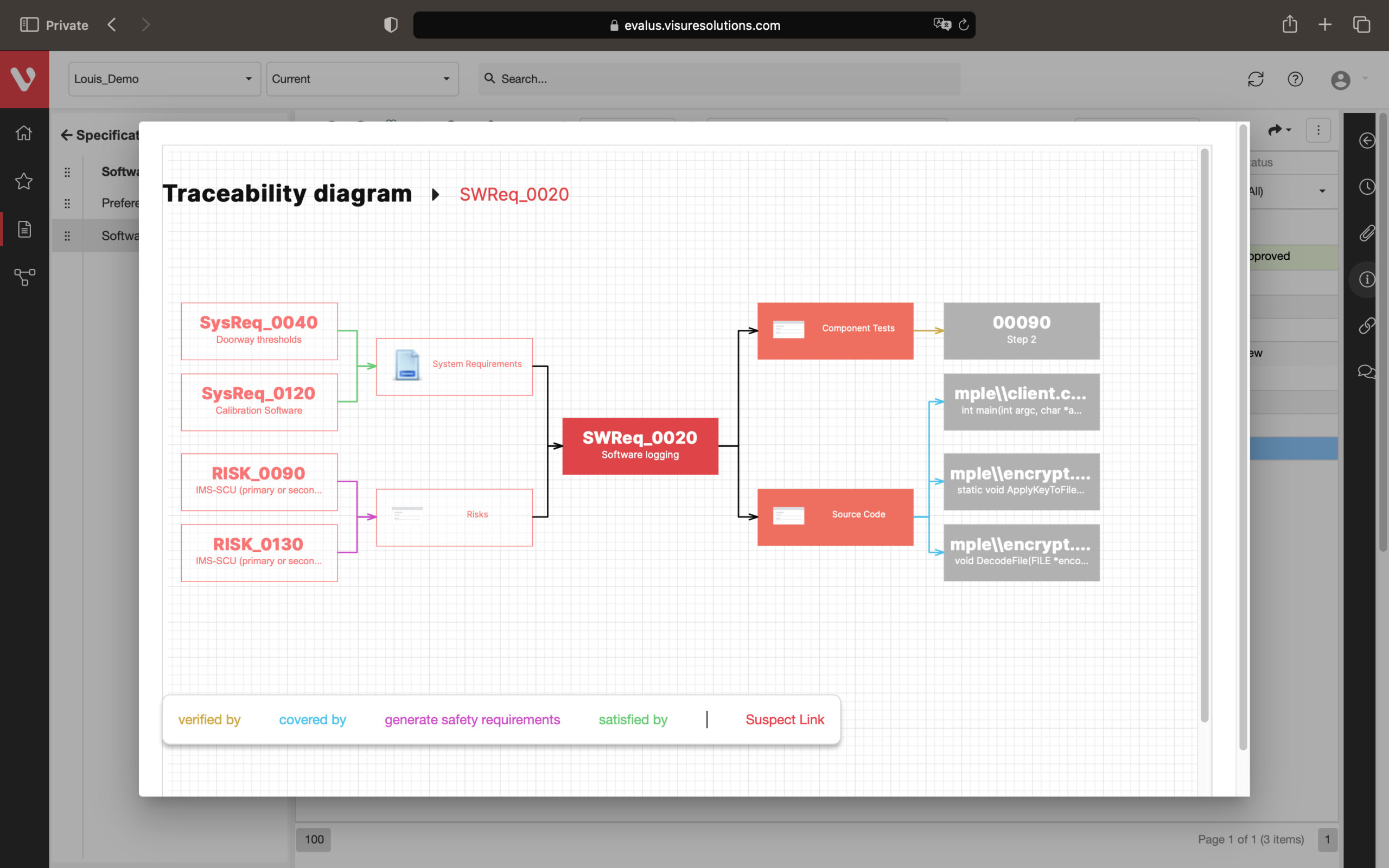
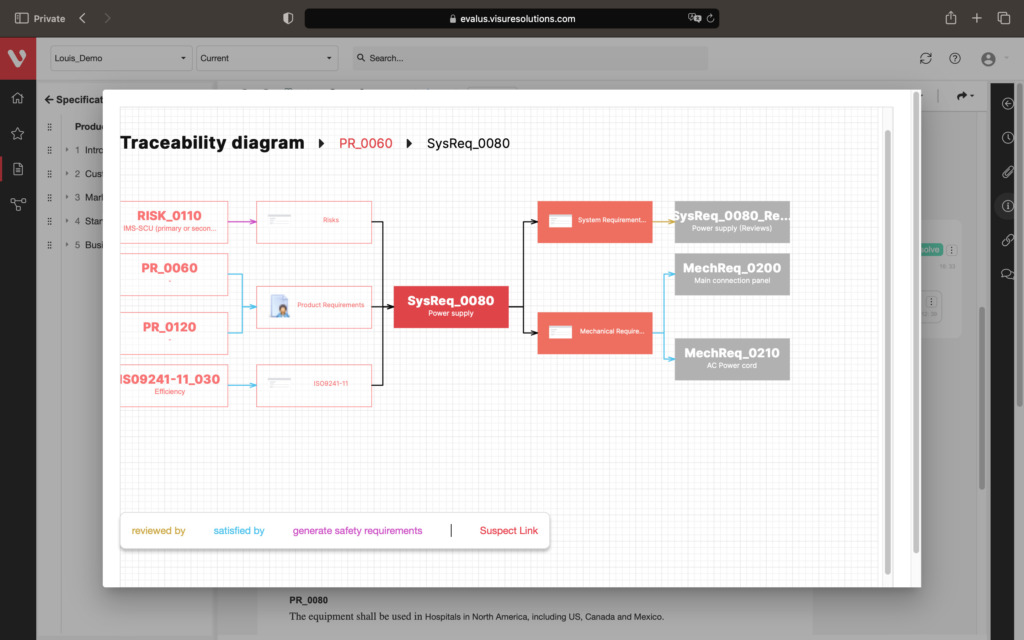
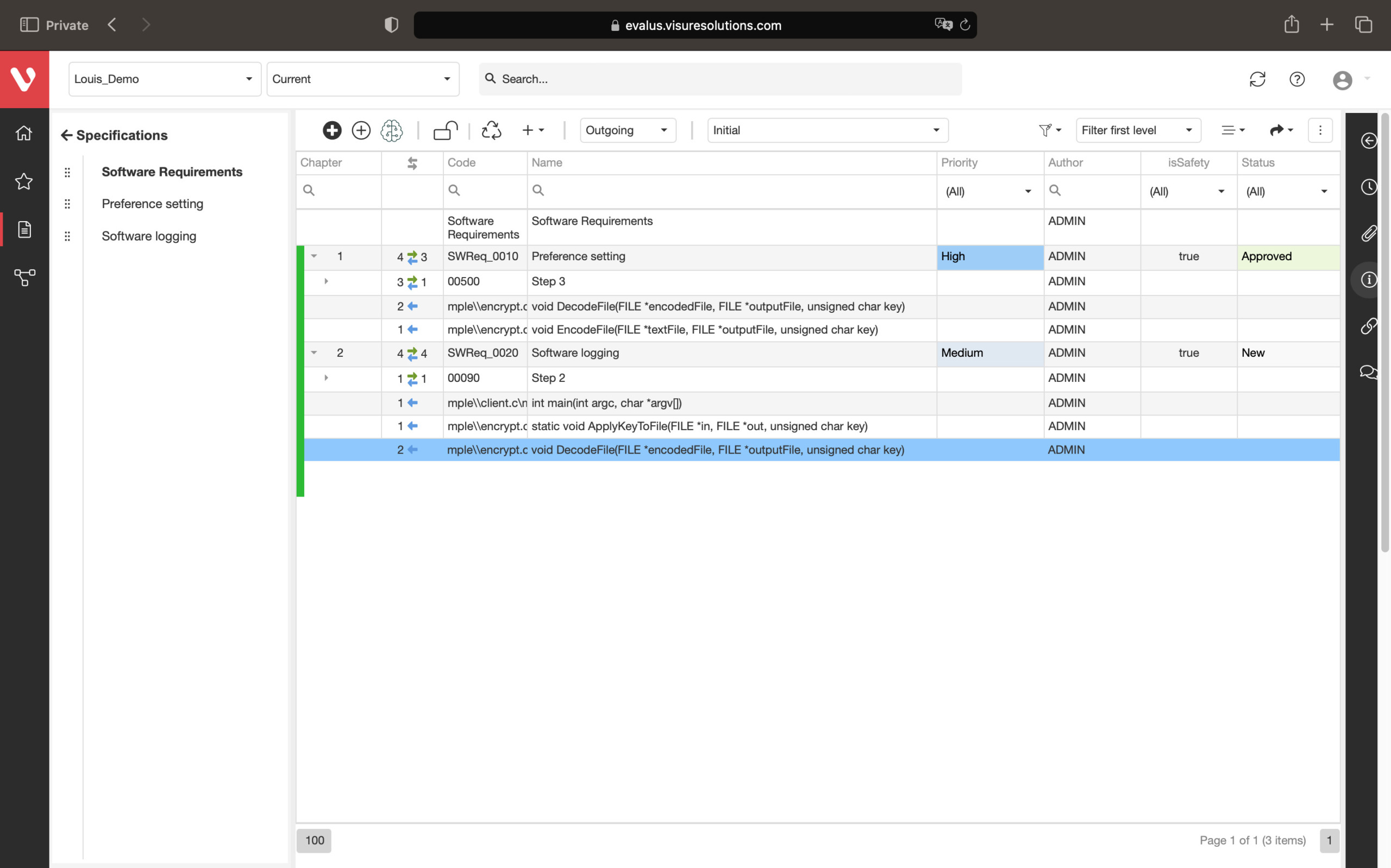
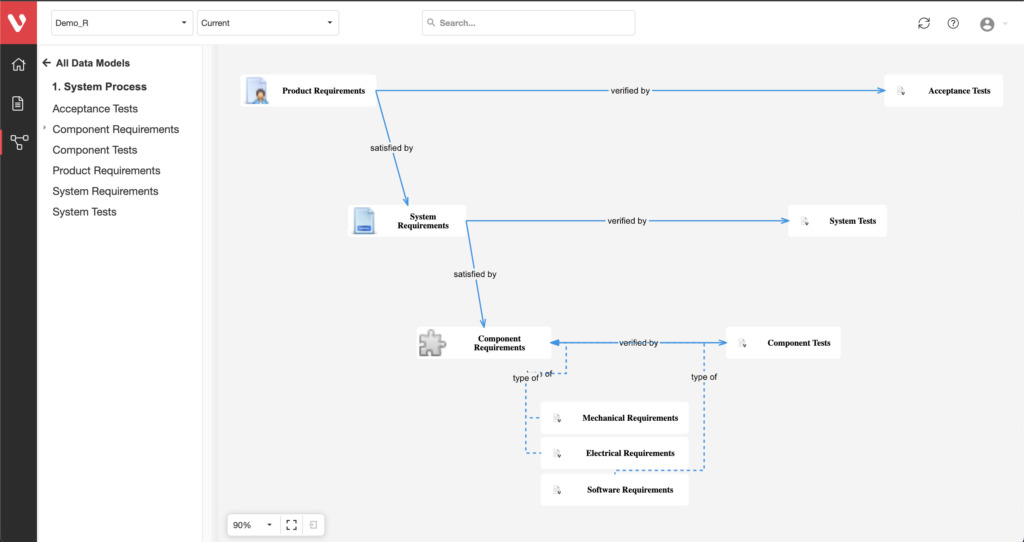
Effortlessly explore upstream and downstream relationships, anticipate change impact, monitor cross-project relationships, detect potential link issues during changes, and visualize relationship rules across projects to understand their organizational impact and reach.
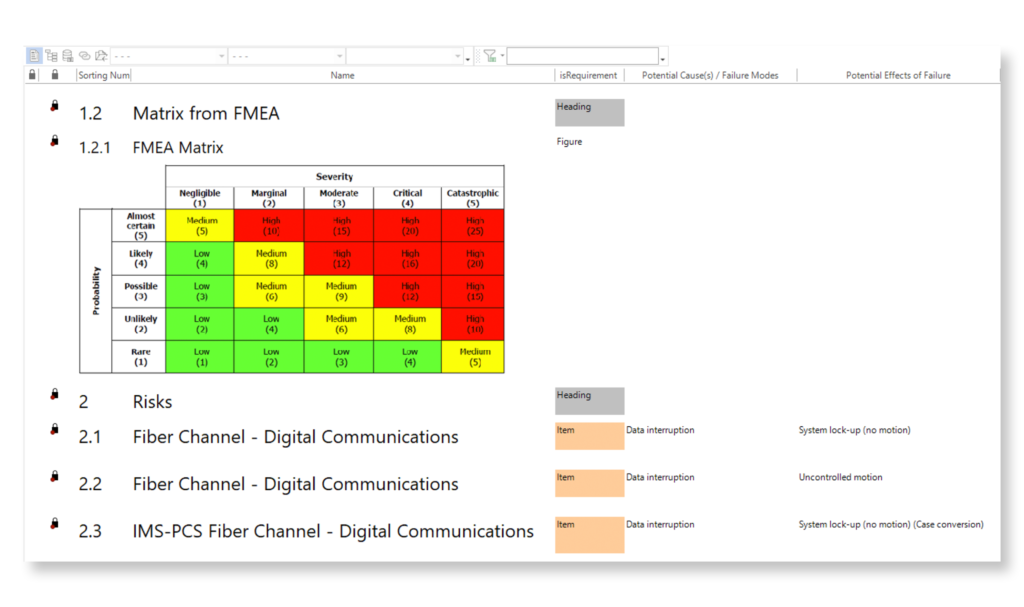
Keep your risk analysis current with real-time data, improve coverage by tracing open risks to requirements, build a comprehensive risk management profile using PHA and FMEA techniques, and guarantee quality and safety in intricate product development.
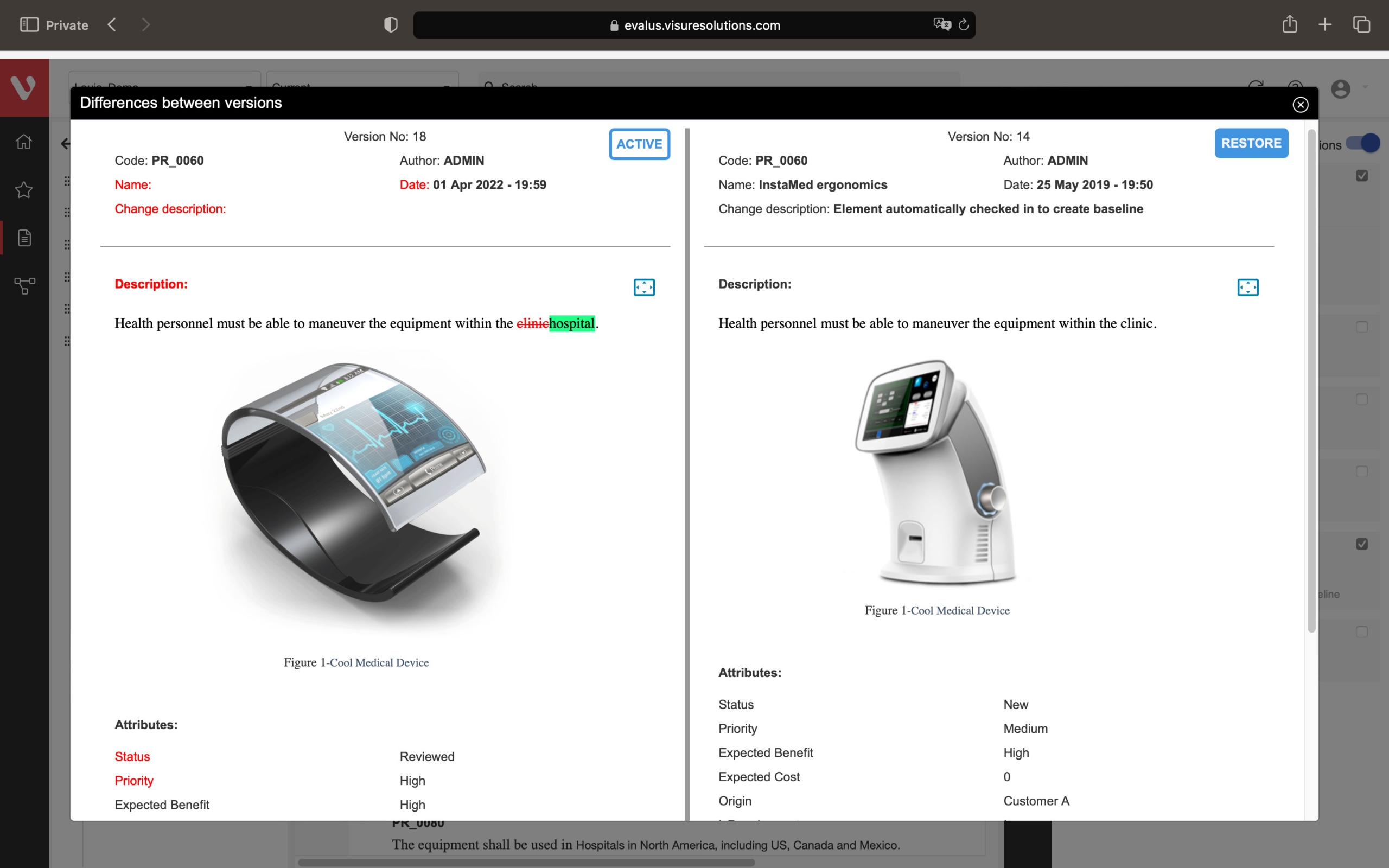
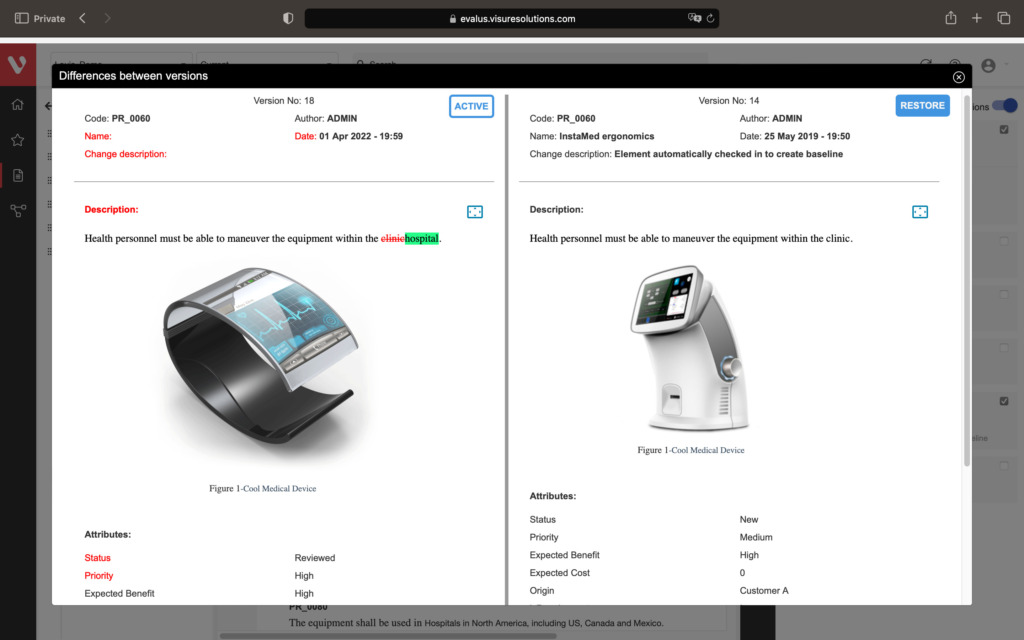
Speed up development and maintain consistency. Easily compare requirement versions, organize and secure your data, and create development stream baselines or requirement catalogs.
Compare changes in requirements, ensure data organization and security, snapshot project states, build reusable requirements catalogs, and develop product variants or new versions.
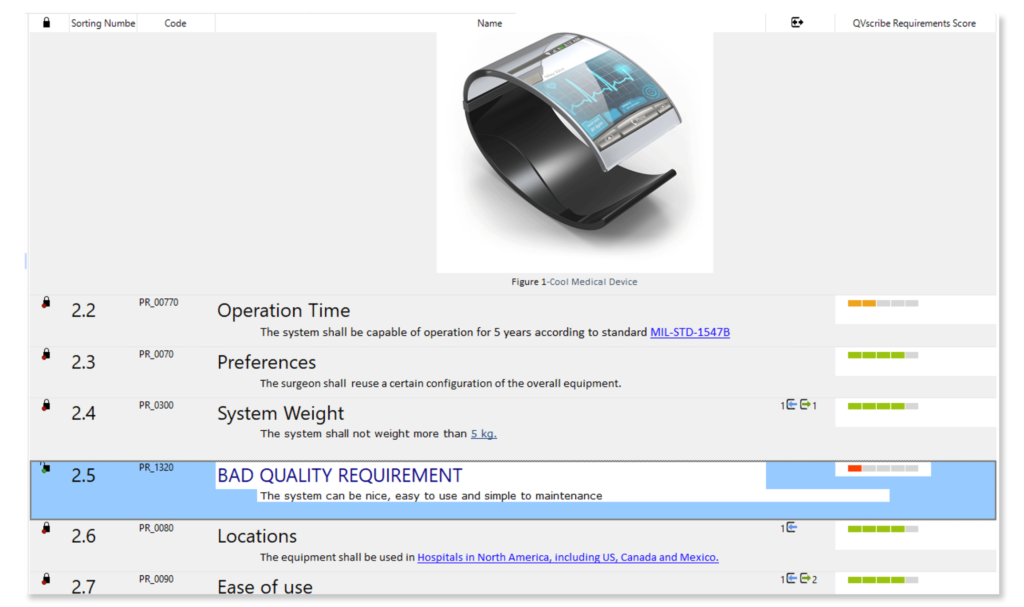
Automatically analyze the quality of requirements while writing them. Avoid ambiguous specifications from poorly written, ambiguous, and inconsistent requirements.

Increase your productivity and ease your Stakeholder review process by using simple import and export data features for ReqIF, and MS Office Word & Excel.

Automate Your Proof of Evidence.
Optimize Your DO-178C & DO-254 Certification Compliance.
With Visure, you can use automated checklists to manage compliance and easily integrate and access our DER partner’s checklists into our tool. This will enable you to design and improve a review process around these checklists.

Why Top Leading Medical Device Companies Choose Visure For IEC 62304 Certification

Reduce Risk & Manage Standard Compliance
Mitigate Risk and avoid stressful compliance audits across projects by centralizing and tracing in a single source of platform.
Full end-to-end Traceability, including Source Code
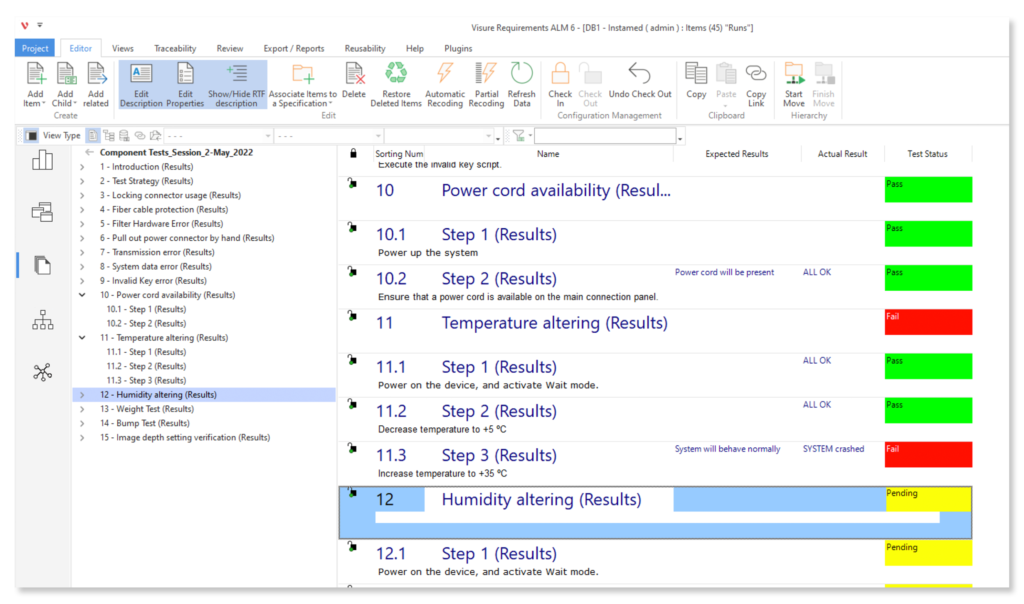
Configure your data model and gain full traceability between tests, requirements, risk, defects and all items, including source code to specific requirements.

Simple Import and Export Data
Increase your productivity by using simple import and export data features from ReqIF and MS Office Word and Excel.

Facilitate Real Time Collaboration & Alignment
Visure integrates bi-directionally and automatically with the top industry engineering tools, easing collaboration among teams in real time.

Easy to use UX/UI Requirements ALM Tool
Forget about legacy tools user friendly experience, and implement an easy to use Requirements ALM tool with a low learning curve.

Most Value to Price Product in the Market Guaranteed
We are committed to your team's project success by delivering within budget. That's why Visure's pricing is a fraction from other competitors.
Maintain Security Across Development
With our On-Premise Licensing option, you can easily deploy and maintain security across all your projects within the tool.

Accelerate Project Speed to Market
Increase your team's productivity with reusability of components across projects and automating repetitive tasks through open source code & AI.

Access Premium Support, Trainings and Consultations
Fast track your team's success by getting your team up and running easily, while staying on top with industry best practices.
Visure Requirements ALM Connects with Best-of-Breed AVIATION SOFTWARE
And even more integrations with other leading software — including automated test solutions— to accelerate and facilitate success across the entire product development lifecycle.
What industry professionals say about us





As posted in G2, SoftwareReviews and TrustRadius.
Ensure IEC 62304 Compliance.
Enforce Traceability.
Ease & Accelerate Your Audits.
- Most cost-effective
- Access All Features
- 30-Day Trial
On average, our customers experience:
See what’s possible with a Modern Medical Devices ALM Software Solution for IEC 62304 certification.
PER PROJECT
TO MARKET
PREPARING FOR AUDITS